Gallery
Photos from events, contest for the best costume, videos from master classes.
 | |
 | |
 |  |
 |  |
 |  |
 | 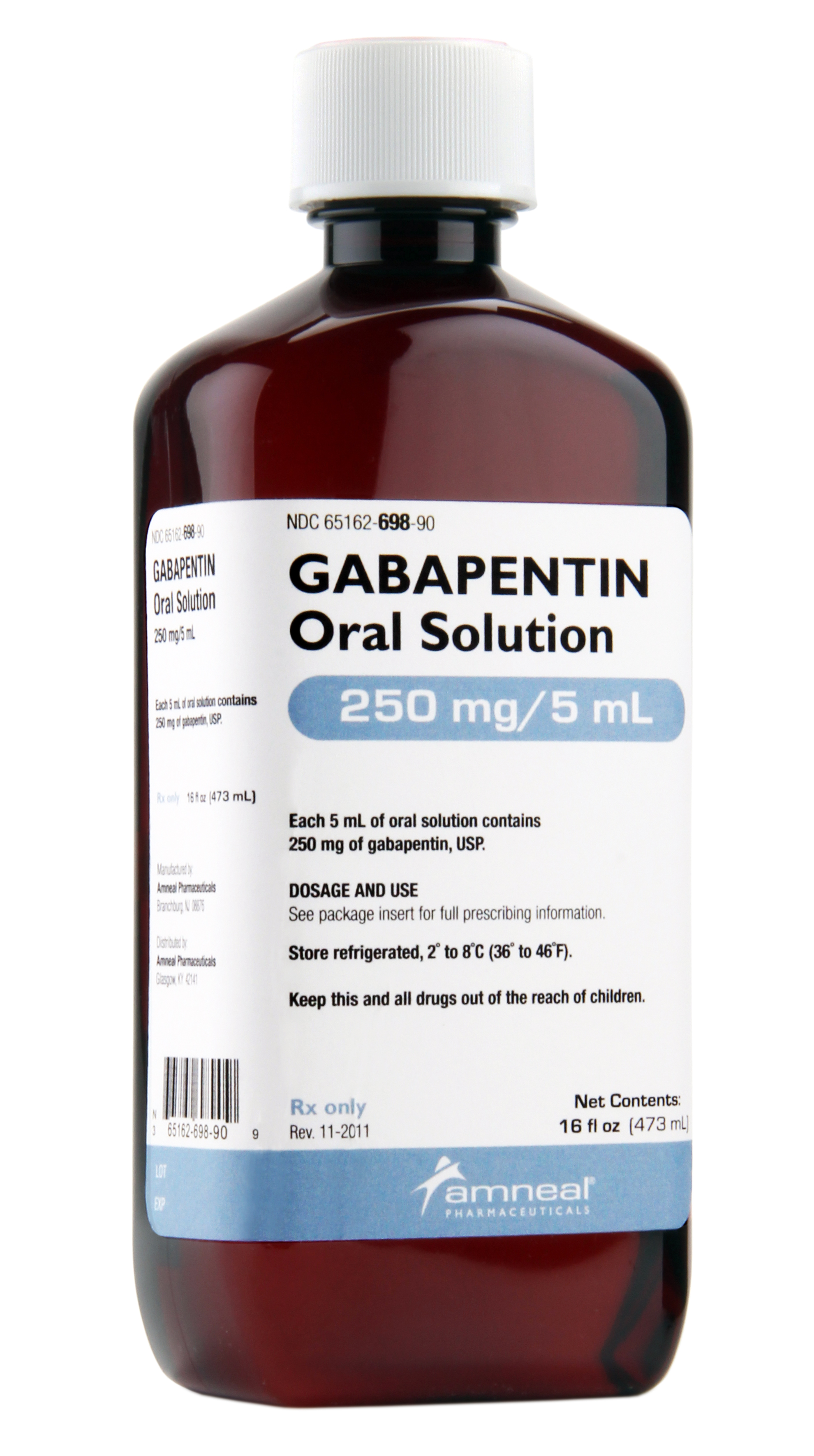 |
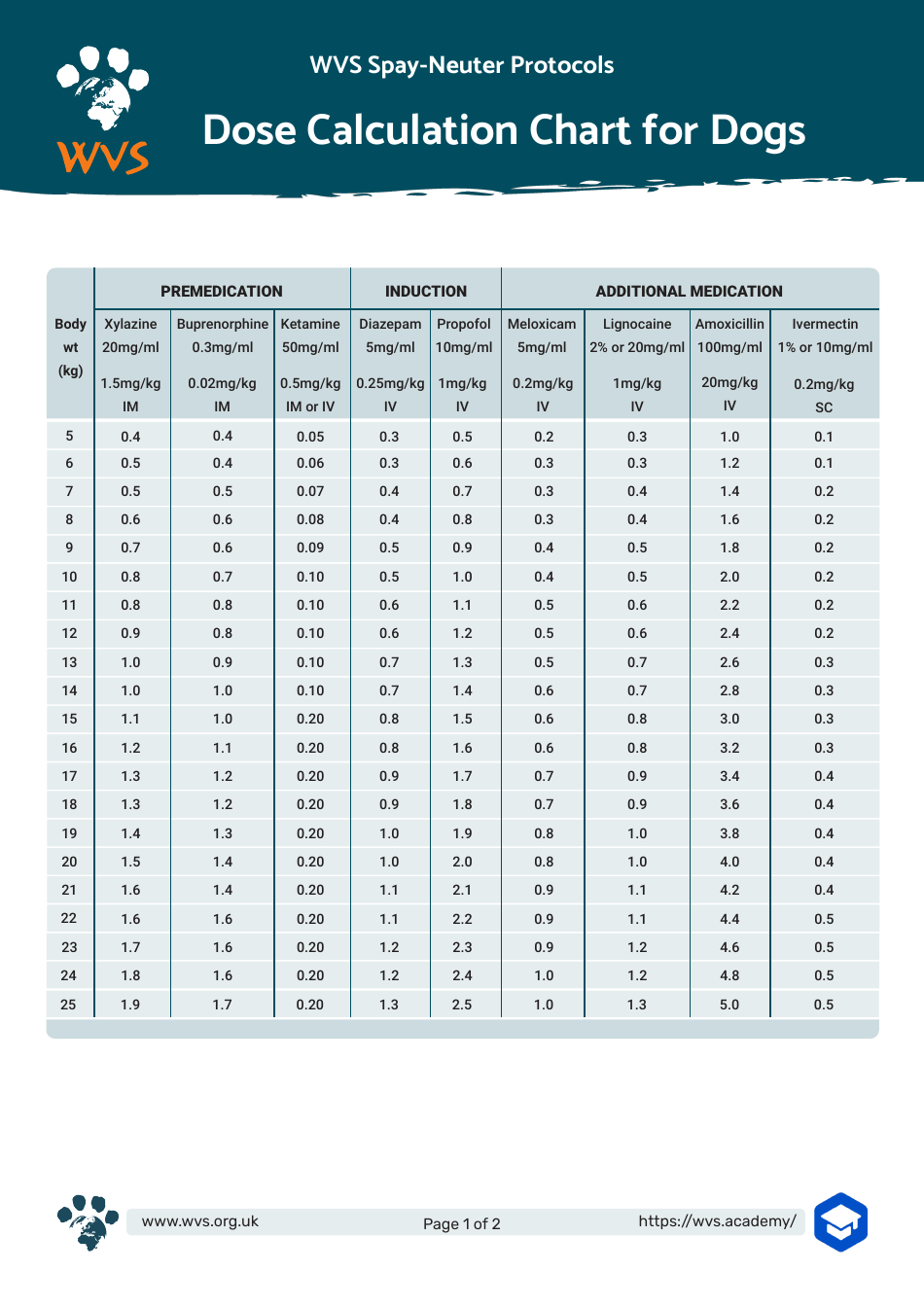
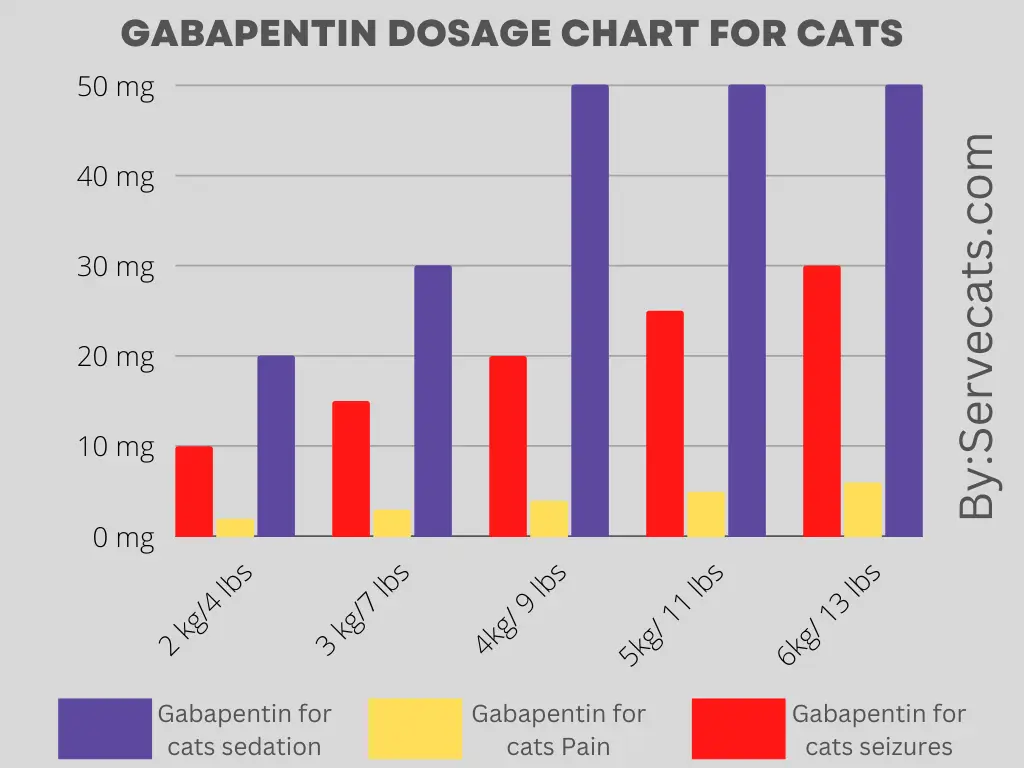
Advagen Pharma Ltd: Gabapentin oral solution is indicated for: Management of postherpetic neuralgia in adults. Adjunctive therapy in the treatment of partial Gabapentin Oral Solution is indicated for: •Epilepsy with Partial Onset Seizures ( 2.2) •Patients 12 years of age and older: starting dose is 300 mg three times daily; may be titrated up to 600 mg three times daily. The active ingredient in gabapentin oral solution is gabapentin, USP, which has the chemical name 1- (aminomethyl)cyclohexaneacetic acid. The molecular formula of gabapentin, USP is C9H17NO2 and DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. For more information about Gabapentin Oral Solution, medical inquiries, or to report side effects regarding Gabapentin Oral Solution, please call 1-800-541-4802. AdvaGen Pharma is focused on delivering value-added healthcare solutions to patients, customers and partners by developing, manufacturing, and marketing high quality branded and generic prescription pharmaceuticals. Gabapentin may be administered as the oral solution, capsule, or tablet, or using combinations of these formulations. Dosages up to 50 mg/kg/day have been well tolerated in a long-term clinical study. The maximum time interval between doses should not exceed 12 hours. Do not take gabapentin oral solution if you are allergic to gabapentin or any of the other ingredients in gabapentin oral solution. See the end of this Medication Guide for a complete list of ingredients in gabapentin oral solution. What should I tell my healthcare provider before taking gabapentin oral solution? Gabapentin Oral Solution is supplied as oral solution containing 250 mg/5 mL of gabapentin. The inactive ingredients are anise flavor natural and artificial, cooling agent flavor artificial, glycerin, purified water, strawberry flavor artificial and xylitol. Complete details for NDC 72888-0104-25 Gabapentin 250 mg/5mL including product information, packaging information, pricing, prescribing information and package photos. When the decision is made to co-prescribe Gabapentin Oral Solution with another CNS depressant, particularly an opioid, or to prescribe Gabapentin Oral Solution to patients with underlying respiratory impairment, monitor patients for symptoms of respiratory depression and sedation, and consider initiating Gabapentin Oral Solution at a low dose. Gabapentin requires a prescription from your veterinarian. This medication treats chronic pain such as pain associated with arthritis and other joint problems. In addition, it can be used to treat some seizure disorders. While the mechanism of action for this medication is unknown, it is believed that gabapentin works by decreasing calcium influx and inhibiting excitatory neurotransmitters Gabapentin Oral Solution (gab-ah-PEN-tin) Read the Medication Guide before you start taking gabapentin and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment. These highlights do not include all the information needed to use GABAPENTIN ORAL SOLUTION safely and effectively. See full prescribing information for GABAPENTIN ORAL SOLUTION. GABAPENTIN oral solution Initial U.S. Approval: 1993 GABAPENTIN- gabapentin solution Advagen Pharma Ltd ---------- MEDICATION GUIDE Quantity Price NDC: 72888-0104-25 MANUFACTURER:ADVAGEN PHARMA LTD Reviews 0 Back Gabapentin oral solution is indicated for: In adults with postherpetic neuralgia, gabapentin oral solution may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). The NDC code 72888-104 is assigned by the FDA to the product Gabapentin which is a human prescription drug product labeled by Advagen Pharma Ltd. The product's dosage form is solution and is administered via oral form. Gabapentin is available as oral capsules of 100 mg, 300 mg, and 400 mg; tablets of 100 mg, 300 mg, 400 mg, 600 mg, and 800 mg; and an oral solution of 50 mg/mL. However, the need often exists for a higher-concentration oral liquid, and if tablets or capsules are used as the drug source, a suspension will result. The NDC Packaged Code 72888-104-25 is assigned to a package of 470 ml in 1 bottle of Gabapentin, a human prescription drug labeled by Advagen Pharma Ltd. The product's dosage form is solution and is administered via oral form. DESCRIPTION Gabapentin oral solution is supplied as an oral solution containing 250 mg/5 mL of gabapentin. The inactive ingredients for the oral solution are anise flavor, artificial strawberry flavor, glycerin, hydrochloric acid, purified water, sodium hydroxide and xylitol.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 | |
 | |
 |  |
 |  |
 |  |
 |  |