Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
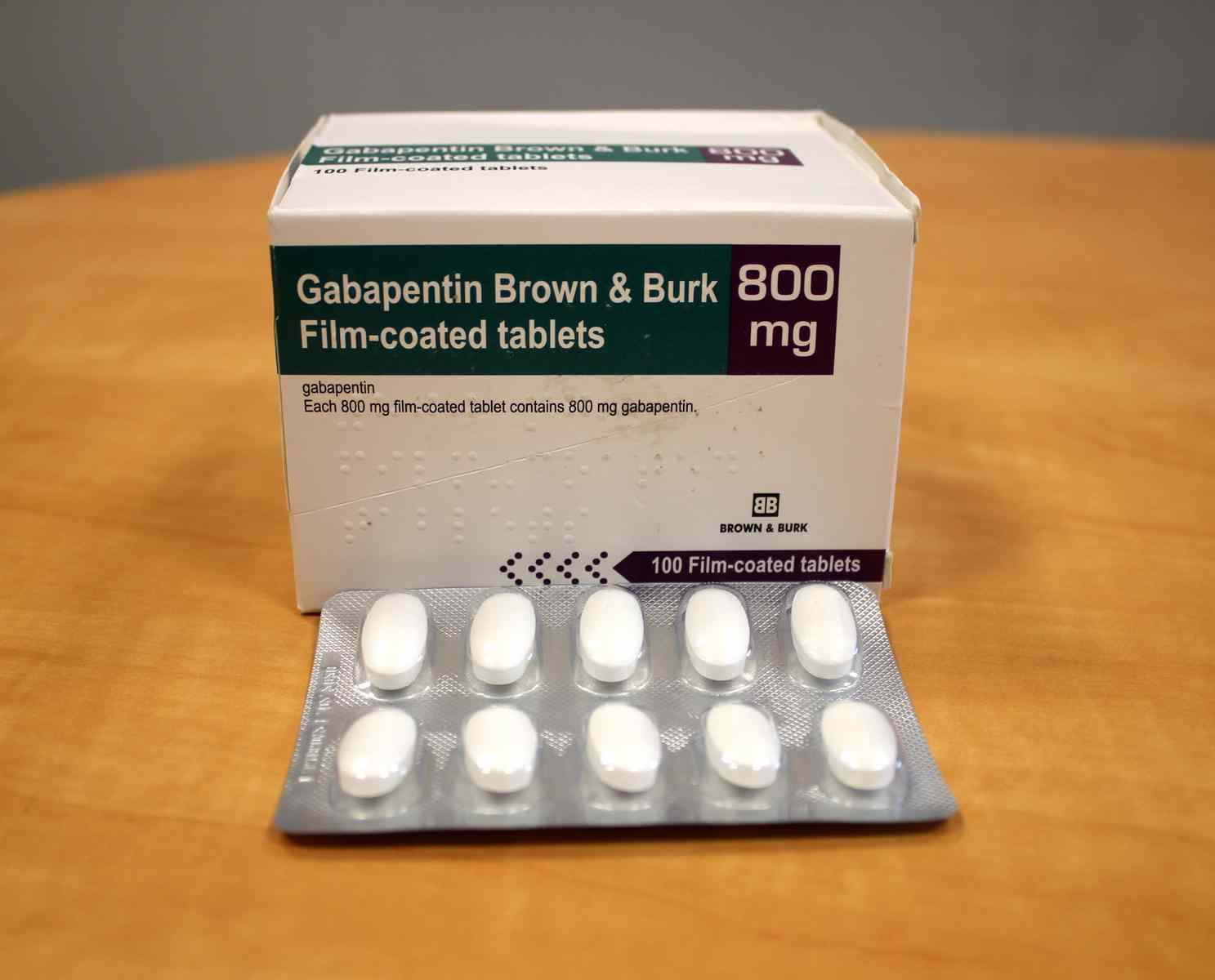 | 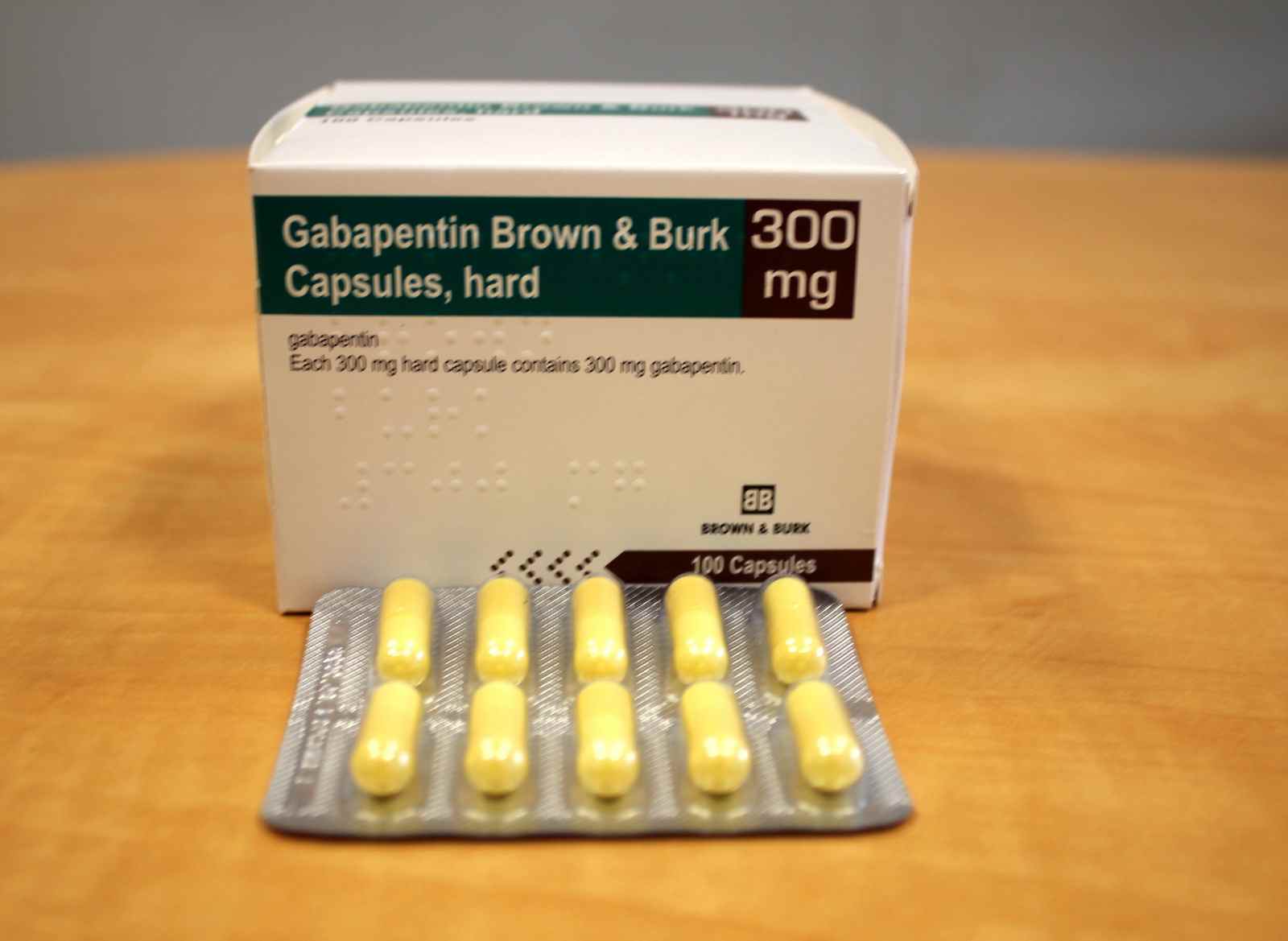 |
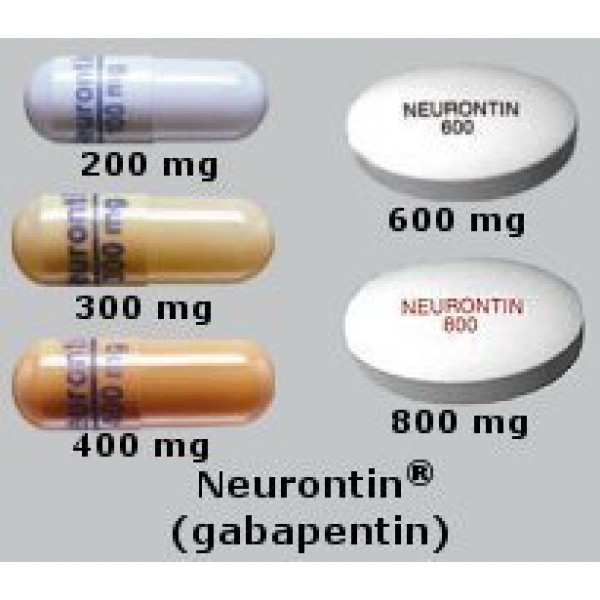
Gabapentin comes as a capsule, a tablet, an extended-release (long-acting) tablet, and an oral solution (liquid) to take by mouth. Gabapentin capsules, tablets, and oral solution are usually taken with a full glass of water (8 ounces [240 milliliters]), with or without food, three times a day. Different forms of gabapentin (such as immediate-release, sustained-release, enacarbil sustained-release) are absorbed in the body differently. Do not switch from one form to the other without consulting your doctor. Others such as "sustained release", “controlled release", etc. are commonly used on package labeling. The term "Slow‐release" is being used here to signify all such drugs with a special‐release mechanism. Gabapentin comes as a capsule, a tablet, an extended-release (long-acting) tablet, and an oral solution (liquid) to take by mouth. Gabapentin capsules, tablets, and oral solution are usually taken with a full glass of water (8 ounces [240 milliliters]), with or without food, three times a day. These medications should be taken at evenly spaced times throughout the day and night; no more than If you are also taking an antacid, it is best to take gabapentin at least 2 hours after taking the antacid. Different forms of gabapentin (such as immediate-release, sustained-release, enacarbil sustained-release) are absorbed in the body differently. Do not switch from one form to the other without consulting your doctor. Immediate-release gabapentin capsules require three times daily dosing (except in kidney disease). 7. Interactions Medicines that interact with gabapentin may either decrease its effect, affect how long it works, increase side effects, or have less of an effect when taken with gabapentin. Gabapentin is a medication used to manage nerve pain (e.g., postherpetic neuralgia), restless leg syndrome, and seizures. Available as gabapentin capsules or extended-release tablets, it calms overactive nerves. EXTENDED RELEASE FORMULATIONS OF GABAPENTIN - Patent 2929878[0005] Immediate-release tablets and capsules of Gabapentin are presently marketed under the trademark Neurontin® as adjunctive orally therapy administered three times per day in the strength of 100mg , 300mg, 400mg, 600mg and 800 mg in the treatment of partial seizures in patients with epilepsy and postherpetic neuralgia. Gabapentin This slow-release gabapentin prodrug reduces International Restless Legs Syndrome (IRLS) Study Group Rating Scale scores and significantly improves subjective sleep quality. Gabapentin has a biological half life of 5-7hours which gives the scope for the development of Controlled release tablets. Controlled release tablets will help to reduce the dose dependency and improve patient compliance.6 Due to its crystalline properties, Gabapentin forms an intact matrix with the high viscous polymers. ABSTRACT Immediate release tablets of Gabapentin were successfully formulated by employing direct compression method. Preformulation studies of drug were performed; the infrared spectral analysis revealed that there is no chemical interaction with excipients used was compatible with drugs. Based on Weight variation, Hardness, friability, and Drug Content it was found that all the formulation Accumulation can cause renal failure resulting in adverse effects. Formulations Gabapentin is available in two extended-release formulations in addition to the immediate release: a gastric retentive formulation (GBP-GR) and a gastro-retentive prodrug gabapentin enacarbil that are approved for the management of postherpetic neuralgia. Includes Gabapentin indications, dosage/administration, pharmacology, mechanism/onset/duration of action, half-life, dosage forms, interactions, warnings, adverse Gabapentin (Neurontin) is a prescription drug. It comes as an oral capsule, an immediate- or extended-release oral tablet, and an oral solution. The pharmacokinetics of gabapentin delivered from this extended-release formulation allows a reduced dosing frequency while maintaining bioavailability and possibly diminishing the occurrence of adverse events attributable to a slower increase to the peak concentration compared with the immediate-release dosage form. Objective: Gabapentin immediate release (GBP-IR), gabapentin gastric retentive (GBP-GR), and the prodrug gabapentin enacarbil extended release formulation (GEn) have been approved for management of postherpetic neuralgia (PHN) in adults. This is the first pharmacokinetic (PK) comparison of all three formulations using FDA-recommended doses for PHN. Gabapentin Extended-Release Tablets What is this medication? GABAPENTIN (GA ba pen tin) treats nerve pain. It works by calming overactive nerves in your body. Horizant (gabapentin enacarbil) is an extended release tablet used to treat restless legs syndrome and for the pain from having shingles (postherpetic nerve pain). Generic brands of gabapentin capsules, USP are used for postherpetic nerve pain and for add on therapy for partial onset seizures in patients 3 years and older Warnings View gabapentin information, including dose, uses, side-effects, renal impairment, pregnancy, breast feeding, monitoring requirements and important safety information. Immediate-release gabapentin has a half life of 5 to 7 hours, but extended-release formulas are designed to release the drug more slowly, extending its action over a longer period and potentially increasing its half life.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 |  |