Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 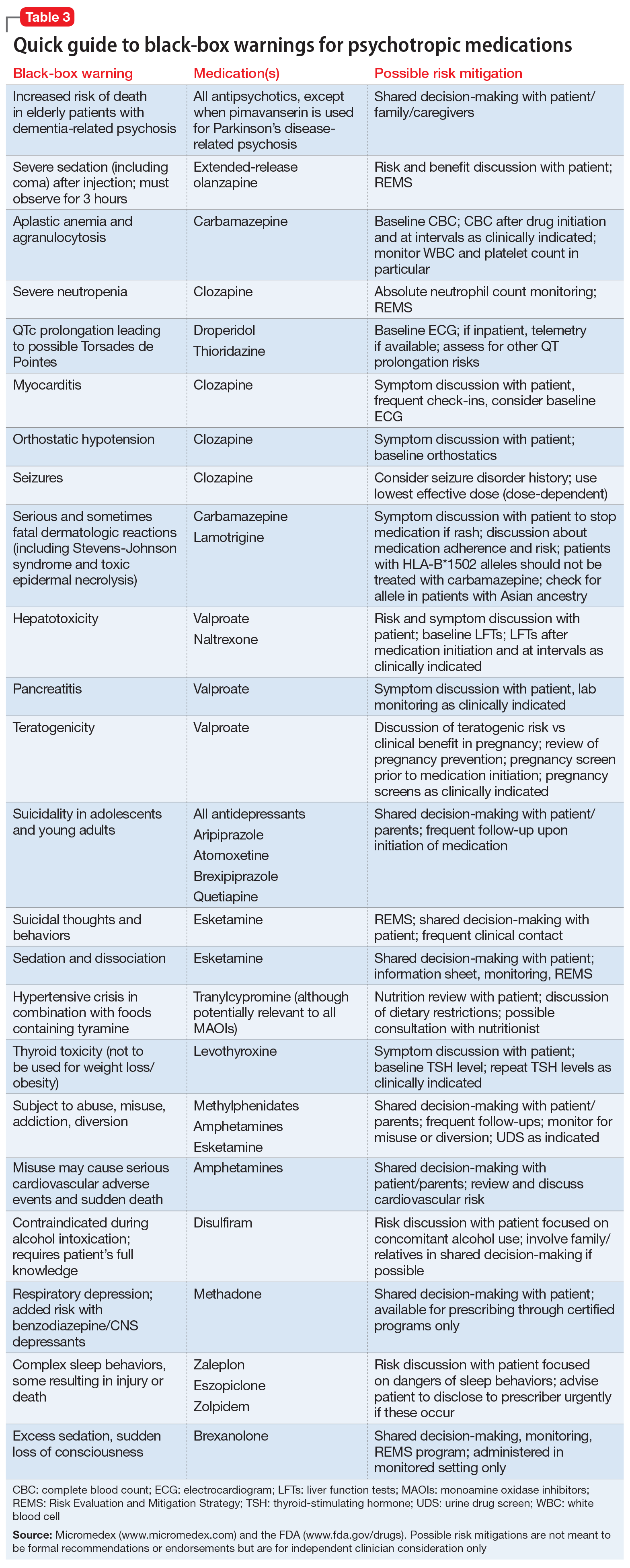 |
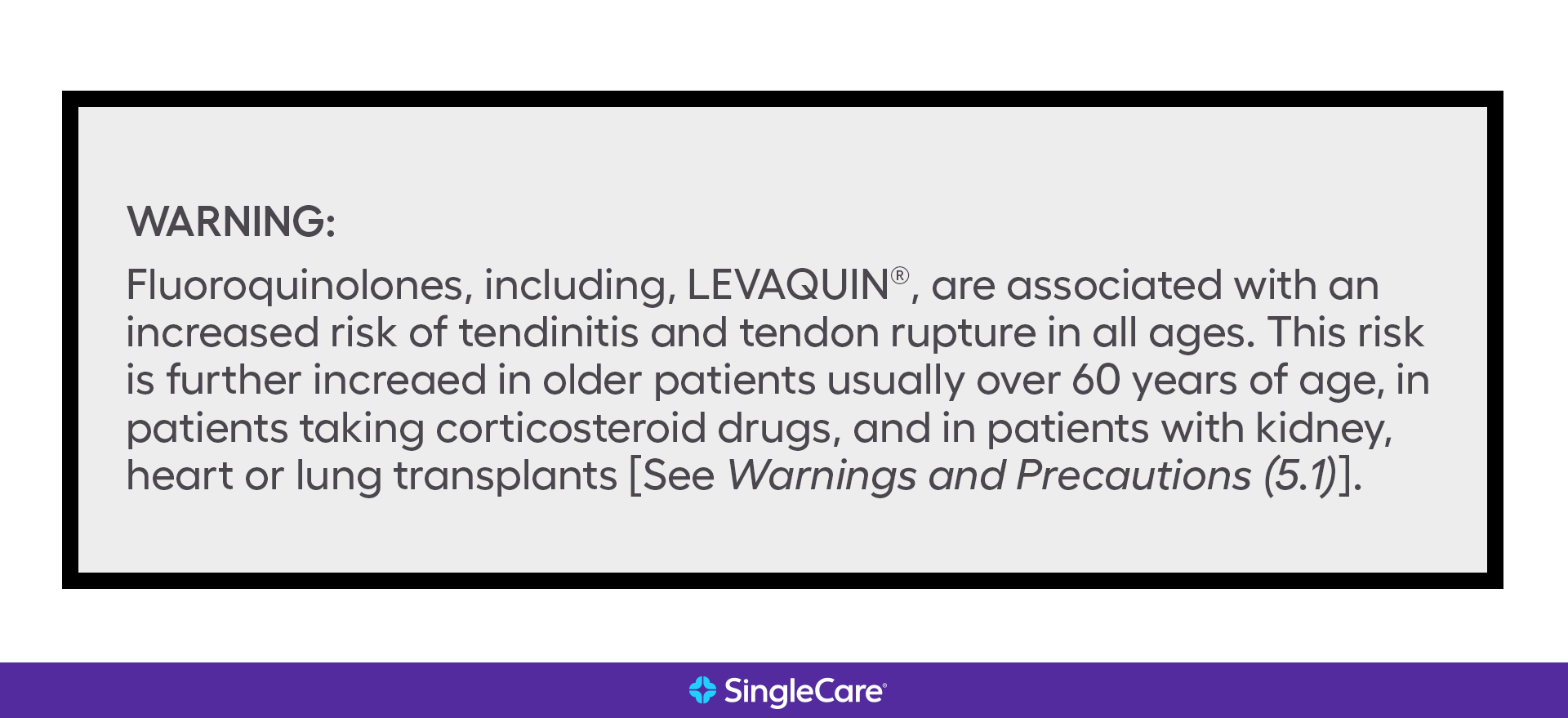
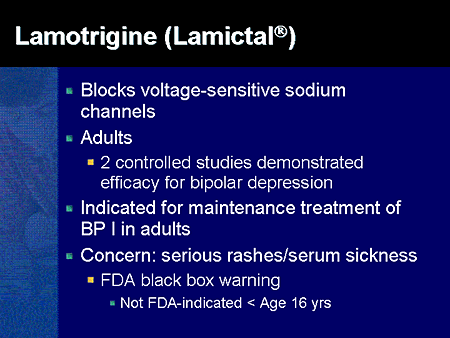
FDA is requiring new warnings about risk of respiratory depression in patients who use gabapentanoids with opioids or drugs that depress the nervous system ISSUE: FDA is warning that serious breathing difficulties may occur in patients using gabapentin (Neurontin, Gralise, Horizant) or pregabalin (Lyrica, Lyrica CR) who have respiratory risk factors. We would like to show you a description here but the site won’t allow us. Gabapentin, opioids, and/or benzodiazepines are commonly prescribed for a variety of pain and psychiatric conditions. Despite the high likelihood of co-prescription of these medications, little is known about co-utilization of gabapentin (GABA), These alerts summarize FDA updates to Prescribing Information for medications used in epilepsy, but may also include relevant news about devices or dietary treatments from other sources. Boxed warnings (formerly known as Black Box Warnings) are the highest safety-related warnings that medications can have assigned by the Food and Drug Administration. These warnings are intended to bring the consumer’s attention to the major risks of the drug. Medications can have a boxed warning added, taken away, or updated throughout their tenure on the market. Over 400 different The FDA recently issued strong gabapentin warnings to doctors. It should not be prescribed with opioids if patients have respiratory problems like COPD. The Boxed Warnings added to pregabalin and gabapentin products advise prescribers to assess a patient's risk of misuse (for pregabalin), and abuse or dependence (for pregabalin and gabapentin) before prescribing these medicines, and to monitor them regularly during treatment. Boxed Warnings for Pregabalin and Gabapentin for drug misuse, abuse and dependence: Safety alert from Australian health authority, TGA. Although a black-box warning was not approved, the FDA collected data on the use of 11 anti-epileptic medications, including gabapentin, between 2005 and 2007 to determine whether there was indeed an increased risk of suicidal ideation or behavior. FDA Issues Warning About Breathing Difficulties With Gabapetinoid Use By staff Washington, DC— Gabapentin and pregabalin are approved for treating patients with several specific neurological and neuropathic conditions. Yet, off-label use of these gabapentinoids has risen astronomically without much verified benefit, according to past research. What Clinicians Need to Know About New FDA Respiratory Warnings on Gabapentin and Pregabalin Products New warnings link this drug class to respiratory depression and abuse potential. Should you be concerned about ‘black box warnings’ on medications? A pharmacist has your answer. The FDA has warned that gabapentin and pregabalin, used to treat a range of conditions including pain and seizures, may cause serious breathing problems. In 2019 the FDA issued a warning about the potential risks of respiratory depression in patients taking gabapentin or pregabalin in combination with central nervous system (CNS) depressants such as opioids, antidepressants, and benzodiazepines. On December 16, 2008 FDA issued a class warning for antiepileptic drugs and suicidal thoughts and behavior. The purpose of this study was to determine if the antiepileptic drug gabapentin increases risk of suicide attempt in patients to which it was That is why a new drug-safety communication from the FDA is so concerning. The agency is warning that serious breathing difficulties may occur in patients using gabapentin (Neurontin, Gralise, Horizant) or pregabalin (Lyrica, Lyrica CR) who have respiratory risk factors. Recalls, warnings, and alerts A warning that serious breathing problems may occur in patients using gabapentin (Neurontin, Gralise, Horizant) or pregabalin (Lyrica, Lyrica CR) who have respiratory risk factors. Risk factors include the use of opioids and other central nervous system depressants and conditions that reduce lung function, such as chronic obstructive pulmonary disease. Elderly Medications affected by this warning include gabapentin (brand names: Neurontin and Gralise), gabapentin enacarbil (a gabapentin pro-drug under the brand name Horizant), pregabalin (brand names: Lyrica and Lyrica CR) and generic versions of gabapentinoids. Listen to an audio podcast of the December 19, 2019 FDA Drug Safety Communication warning that serious breathing difficulties may occur in patients using seizure and nerve pain medicines
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |