Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 | :max_bytes(150000):strip_icc()/kidney-disease-causes-5b2a74c38023b900377a00d3.png) |
 |  |
 | 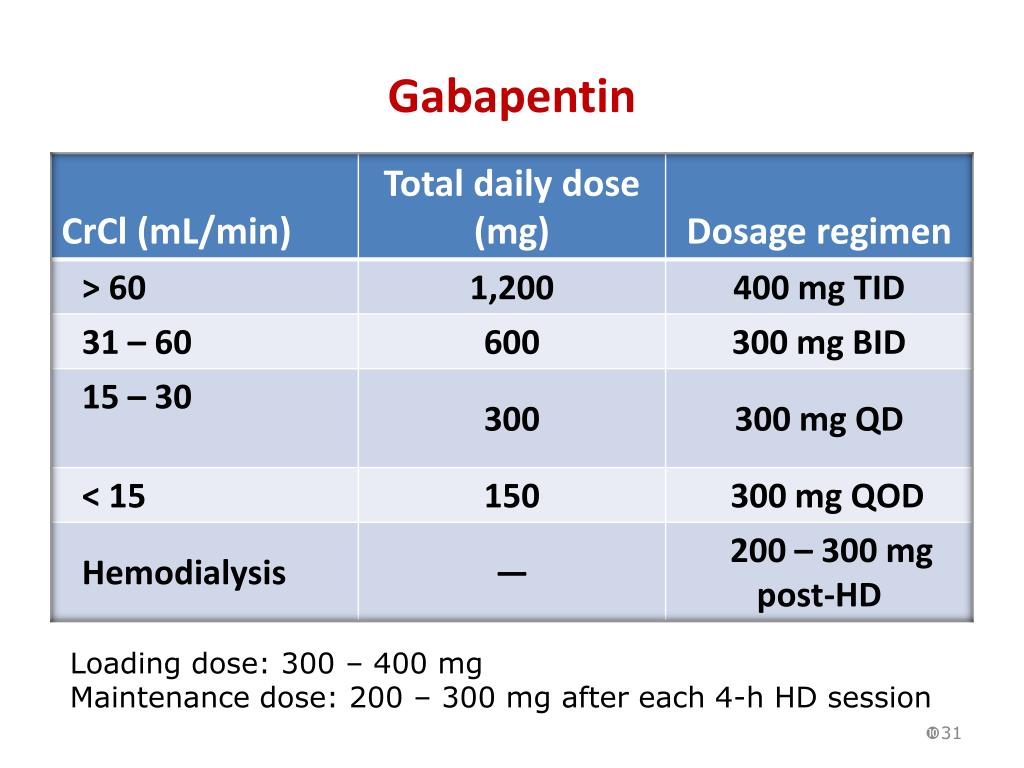 |
For patients with normal kidney function, Gabapentin's half-life—approximately 5 to 7 hours—allows for effective dosing without significant accumulation. However, those with compromised renal function may experience prolonged effects due to slower elimination rates. Although gabapentin is well known for its well recieved pharmacokinetics, it is exclusively eliminated renally, and patients with chronic kidney disease are at risk for toxicity. Gabapentinoids are opioid substitutes whose elimination by the kidneys is reduced as kidney function declines. To inform their safe prescribing in older adults with chronic kidney disease (CKD), we examined the 30-day risk of serious adverse events according to the prescribed starting dose. The FDA drug label does not directly answer whether gabapentin worsens renal function. However, it does indicate that gabapentin is substantially excreted by the kidney and that patients with impaired renal function are at a greater risk of toxic reactions. We found that patients with chronic kidney disease had elevated serum gabapentin concentrations, in some cases leading to gabapentin toxicity; those with advanced age and multiple comorbidities were more prone to the toxicity, and the toxicity tended to be underrecognized. Key takeaways: Gabapentin (Neurontin, Horizant, Gralise) usually isn’t bad for your liver or kidneys. In most cases, it has no harmful effect on these organs. In rare instances, gabapentin can cause DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome. Gabapentin contains a cyclohexyl group and is a form of gamma-aminobutyric acid (GABA). Despite its name, gabapentin does not affect the inhibitory neurotransmitter GABA or its receptors. Instead, it acts as a ligand, binding strongly to the α2δ Gabapentin is frequently used as an analgesic in patients with chronic kidney disease. Although gabapentin is well known for its favorable pharmacokinetics, it is exclusively eliminated renally, and patients with chronic kidney disease are at risk for toxicity. Existing literature on such risk is lacking. INTRODUCTION Pain is one of the most common and distressing symptoms among patients with chronic kidney disease (CKD) [1]. The prevalence of pain has been associated with substantially lower health-related quality of life and greater psychosocial distress, insomnia, and depressive symptoms [2-9]. Abstract Background: Gabapentinoids (GPs) are frequently prescribed in individuals with chronic kidney disease (CKD); however, their exclusive renal elimination warrants dose adjustments to decrease risk of toxicity. This study evaluated GP prescribing patterns and whether excessive dosing was associated with increased incidence of gabapentinoid-related adverse events (GRAEs). Gabapentin’s apparent total clearance is 100 mL/min in adults with normal renal function, which is essentially equivalent to CrCl and does not suggest the involvement of tubular reabsorption. 1 Some evidence suggest that active tubular secretion mediated by organic cation transporter-1 (OCT-1) may play a role in gabapentin’s renal clearance. Gabapentin is frequently used as an analgesic in patients with chronic kidney disease. Although gabapentin is well known for its favorable pharmacokinetics, it is exclusively eliminated renally, and patients with chronic kidney disease are at risk for toxicity. Existing literature on such risk is lacking. CONCLUSION: Gabapentin toxicity in patients with chronic kidney disease is underrecognized. Patients with chronic kidney disease often receive inappropriately high gabapentin dosage for their kidney function, occasioning overt toxicity; advanced age and comorbidity predispose these patients for toxicity. Gabapentin should be used with caution for animals with liver or kidney disease, as it will take longer to metabolize. Gabapentin is available in several forms that are human-labeled products: Gabapentin is frequently used as an analgesic in patients with chronic kidney disease. Although gabapentin is well known for its favorable pharmacokinetics, it is exclusively eliminated renally, and patients with chronic kidney disease are at risk for toxicity. Existing literature on such risk is lacking. In summary, we can conclude that although it happens infrequently, gabapentin may cause myotoxicity, rhabdomyolysis and renal failure even in patients whose renal function was previously normal. For people with normal kidney function, gabapentin is safe and doesn’t cause kidney complications or trigger kidney disease. In people with renal impairment, gabapentin can be harder to clear from the body. Abstract Background: Gabapentin is frequently used as an analgesic in patients with chronic kidney disease. Although gabapentin is well known for its favorable pharmacokinetics, it is exclusively eliminated renally, and patients with chronic kidney disease are at risk for toxicity. Existing literature on such risk is lacking. Gabapentin is widely used in the management of pain. It is entirely excreted through the renal system so this needs to be considered in any patient becoming acutely ill and developing renal failure. We describe a patient who developed significant deterioration in her conscious level due to iatrogenic gabapentin overdose. Conclusion. Discussion: Gabapentin is widely used in the management of pain. It is entirely excreted through the renal system so this needs to be considered in any patient becoming acutely ill and developing renal failure.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 | :max_bytes(150000):strip_icc()/kidney-disease-causes-5b2a74c38023b900377a00d3.png) |
 |  |
 |  |