Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
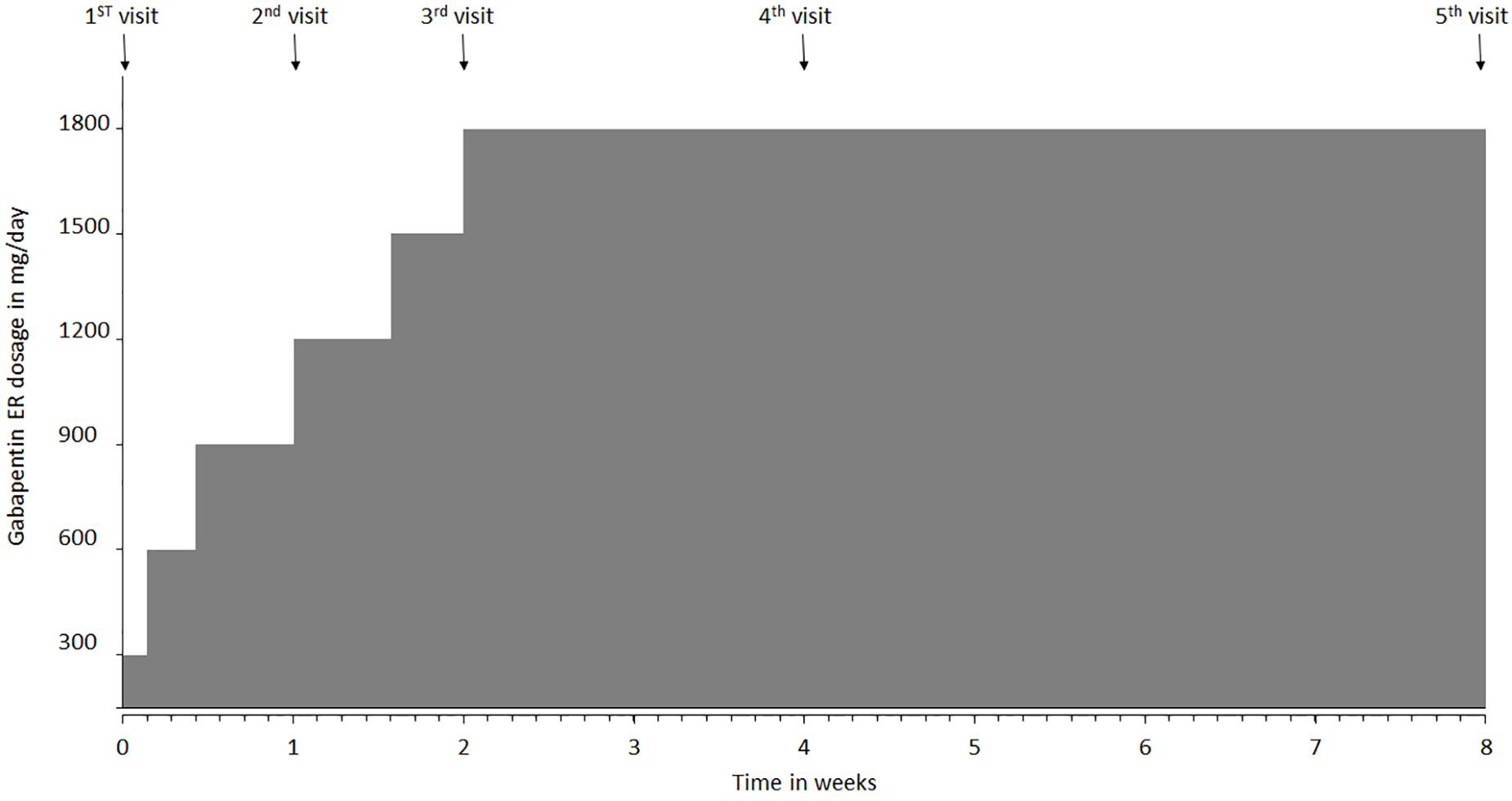
Introduction: Gabapentin is a prescription medication approved by the United States Food and Drug Administration (FDA) for the treatment of neuropathic pain and epileptic disorders. This drug is currently marketed in capsule, tablet, and oral solution formulations. In recent years, however, gabapentin has been increasingly encountered by law enforcement, documented in national crime lab Advise the patient to read the FDA-approved patient labeling (Medication Guide). Administration Information - Inform patients that gabapentin capsules are taken orally with or without RX DRUG SCHEDULING & MONITORING Prescription Drug Monitoring Programs (PDMPs) are electronic databases that collect information on the dispensing and prescribing of drugs within jurisdictions. PDMPs aim to assist patients in their quality of care by allowing prescribers and dispensers access to the patient’s controlled substance prescription medication history. This access to individual Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. However, it was later discovered that gabapentin has the potential of an anticonvulsive medication and can be used as an adjunct to more Gabapentin and pregabalin are FDA-approved for a variety of uses include fibromyalgia and restless legs syndrome. Gabapentin was first approved in 1993 and pregabalin was Gabapentin isn't a narcotic, but it is a controlled substance in some states. Here's what you should know before using it. Drug Schedules Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules depending upon the drug’s acceptable medical use and the drug’s abuse or dependency potential. The abuse rate is a determinate factor in the scheduling of the drug; for example, Schedule I drugs have a high potential for abuse and the potential to create A consumer watchdog group has filed a petition with the FDA and DEA demanding regulation as a way to stop abuse and diversion of the anticonvulsant gabapentin. Between August 2016 and July 2018, three states classified gabapentin as a Schedule V drug and nine states implemented prescription drug monitoring program (PDMP) regulation for gabapentin. It is highly unusual for states to take drug regulation Individuals at the highest risk for abusing gabapentin include those with opioid abuse, mental illness, or previous history of prescription drug abuse. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. Unless specifically excepted or unless listed in another schedule, a material, compound, mixture, or preparation that contains any quantity of the following hallucinogenic substances or that contains any of the substance's salts, isomers, and salts of isomers if the existence of the salts, isomers, and salts of isomers is possible within the specific chemical designation (for the purposes of Individuals at the highest risk for abusing gabapentin include those with opioid abuse, mental illness, or previous history of prescription drug abuse. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. Regional Variation Gabapentin’s regulatory status varies by state. Some states classify it as a Schedule V controlled substance due to concerns about misuse and its involvement in the opioid crisis. Others do not schedule it but require mandatory reporting to state prescription drug monitoring programs (PDMPs) to track prescribing and dispensing. Key takeaways: Gabapentin (Neurontin) is FDA-approved to treat specific types of nerve pain and seizures. It’s also sometimes used to treat other health conditions. These include restless leg syndrome, anxiety, and alcohol withdrawal. Gabapentin isn’t a controlled substance according to the federal government. But several states have passed their own laws classifying gabapentin a schedule FDA warns about serious breathing problems with seizure and nerve pain medicines gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica CR) When used with CNS depressants or in Gabapentin is a prescription medication approved by the FDA for the treatment of neuropathic pain (postherpetic neuralgia) and seizure disorders. Why is gabapentin controlled in some states? Gabapentin is structurally and pharmacologically related to pregabalin (Lyrica, Lyrica CR), which is a Schedule V drug and controlled federally in all states. View gabapentin information, including dose, uses, side-effects, renal impairment, pregnancy, breast feeding, monitoring requirements and important safety information. DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. “FDA warns about serious breathing problems with seizure and nerve pain medicines gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica CR)”. When prescribing gabapentin carefully evaluate patients for a history of drug abuse and observe them for signs and symptoms of gabapentin misuse or abuse (e.g., development of tolerance, self-dose escalation, and drug-seeking behavior).
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |