Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
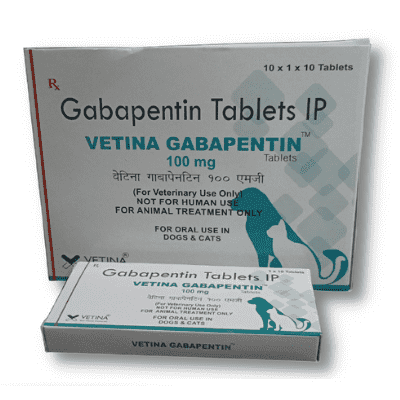
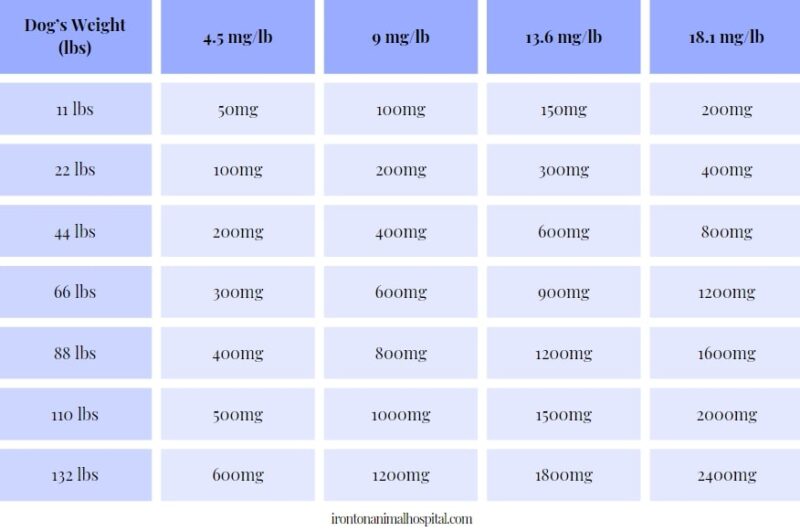
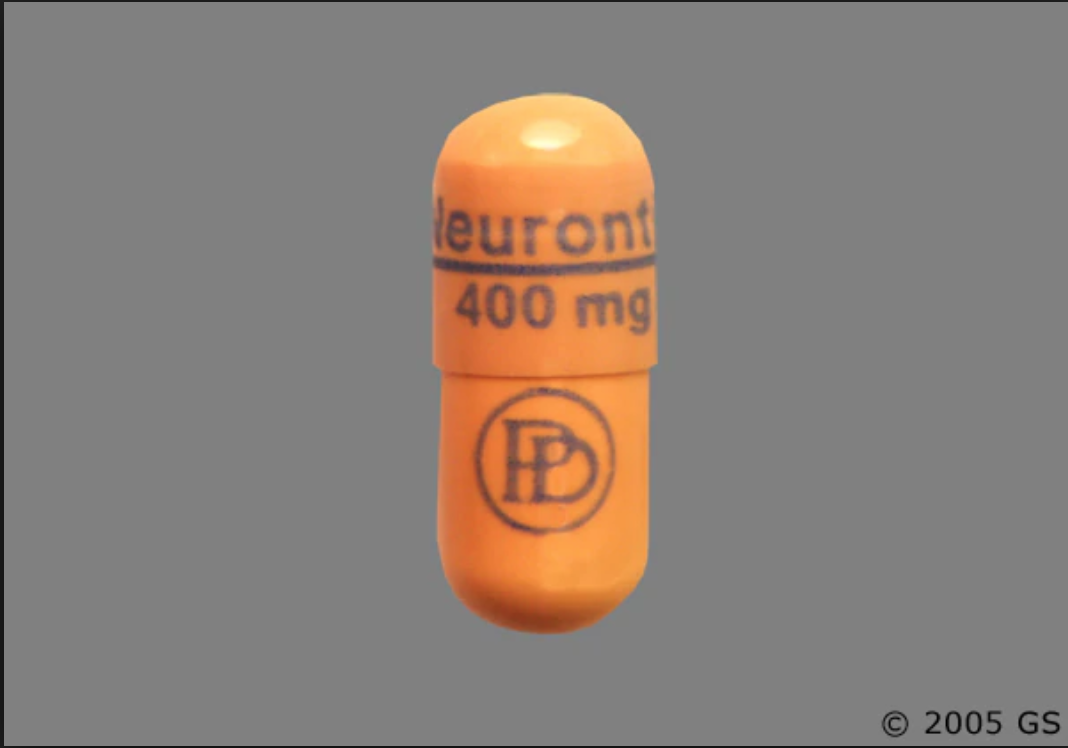
In adults with postherpetic neuralgia, NEURONTIN may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg Pfizer Inc believes that the information contained in this Safety Data Sheet is accurate, and while it is provided in good faith, it is without warranty of any kind, expressed or implied. DATA SHEET NEURONTIN® (gabapentin) 100 mg, 300 mg and 400 mg Capsules 600 mg and 800 mg Tablets Material Name: Gabapentin Capsules (100 mg, 300 mg and 400 mg) Trade Name: NEURONTIN; NEUGABA; GANTIN; PARKETIN; GABAPENTIN PFIZER Coadministration of gabapentin (125 to 500 mg; N=48) decreases hydrocodone (10 mg; N=50) Cmax and AUC values in a dose-dependent manner relative to administration of hydrocodone alone; Cmax and AUC values are 3% to 4% lower, respectively, after administration of 125 mg gabapentin and 21% to 22% lower, respectively, after administration of 500 Source of data R.T.E.C.S. - REGISTRY OF TOXIC EFFECTS OF CHEMICAL SUBSTANCES A.C.G.I.H. - AMERICAN CONFERENCE OF INDUSTRIAL HYGIENISTS H.S.D.B. - HAZARDOUS SUBSTANCES DATA BANK N.I.O.S.H. - NATIONAL INSTITUTE FOR OCCUPATIONAL SAFETY AND HEALTH N.T.P. - NATIONAL TOXICOLOGY PROGRAM I.A.R.C. - INTERNATIONAL AGENCY FOR RESEARCH ON CANCER At 2000 mg/kg, the plasma gabapentin exposure (AUC) in mice is approximately 2 times that in humans at the MRHD of 3600 mg/day. In rats, increases in the incidence of pancreatic acinar cell adenoma and carcinoma were found in male rats receiving the highest dose (2000 mg/kg), but not at doses of 250 or 1000 mg/kg/day. Gabapentin can be used in cats and dogs to help treat epilepsy, anxiety issues (such as going to the veterinary clinic), chronic pain disorders and neuropathic pain. The information and recommendations in this safety data sheet are, to the best of our knowledge, accurate as of the date of issue. Nothing herein shall be deemed to create any warranty, express or implied. The absolute bioavailability of gabapentin following daily doses of 1200 mg/day, 2400 mg/day, 3600 mg/day, and 4800 mg/day averaged 47%, 34%, 33%, and 27% respectively. Material Name: Neurontin (gabapentin) Oral Solution Trade Name: Chemical Family: NEURONTIN Mixture Relevant Identified Uses of the Substance or Mixture and Uses Advised Against Intended Use: Pharmaceutical product used as anticonvulsant Add each solvent one by one: PBS Solubility: 25 mg/mL (145.99 mM); Clear solution; Need ultrasonic and warming and heat to 60°C. Gabapentin is a potent, orally active P/Q type Ca2+ channel blocker. Gabapentin inhibits neuronal Ca2+ influx and reduction. of neurotransmitter release. Show this safety data sheet to the doctor in attendance. Immediate medical attention is required. Rinse immediately with plenty of water, also under the eyelids, for at least 15 minutes. In the case of contact with eyes, rinse immediately with plenty of water and seek medical advice. The recommended maintenance dose of gabapentin in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. Gabapentin may be administered as the oral capsule. Pfizer Inc believes that the information contained in this Material Safety Data Sheet is accurate, and while it is provided in good faith, it is without a warranty of any kind, expressed or implied. GAB with initial concentration of 100 mg L(-1) was eliminated by 80% after 128 min of direct UV irradiation, but just 9% of non-purgeable organic carbon (NPOC) was removed indicating the formation of dead-end transformation products (TPs). DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. Gabapentin can be given orally with or without food. In controlled clinical trials, the effective dose range was 900 mg/day to 1800 mg/day given in divided doses (three times a day). Titration to an effective dose can take place rapidly, over a few days, by giving 300 mg Gabapentin on Day 1, 300 mg gabapentin twice a day on Day 2, and 300 mg gabapentin three times a day on Day 3. Titration may be preferable for patients with renal impairment, patients with encephalopathy, patients on more than 2 other anti-epileptic medications and patients with multiple other medical Synonym(s): Not Applicable Chemical Name: 2-[1-(amino methyl) cyclohexyl] acetic acid Trade Name(s): Gabapentin Capsules, USP 100 mg, 300 mg and 400 mg. Therapeutic Category: Used to relieve pain, especially neuropathic pain, Anticonvulsant. Molecular formula: C9H17NO2 Molecular Weight: 171.24
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |