Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
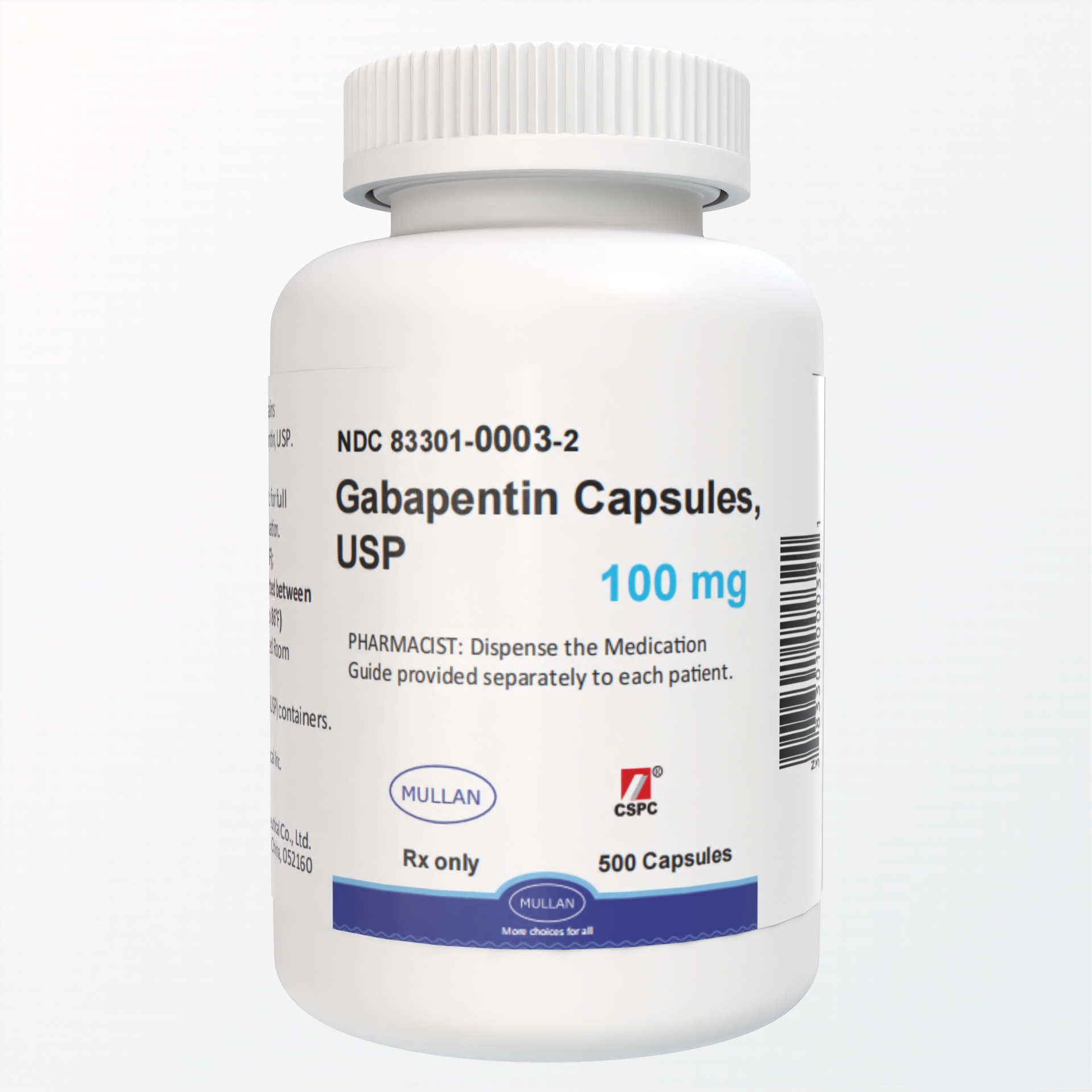
USP 35 Procedure—Separately inject equal volumes (about 20 mL) of the Standard solution and the Test solution into the chromato- graph, record the chromatograms, and measure the responses for the major peaks. [ NOTE—Disregard all the peaks having rela- tive retention times of 0.35 or less relative to gabapentin re- lated compound D, as these are quantified in the test for Limit of Early Test specimen—Grind at least 20 Tablets to a fine powder. Use an amount of powder, equivalent to about 2 mg of gabapentin, and about 200 mg of dry potassium bromide. Gabapentin Capsules, USP 300 mg are available for oral administration as hard gelatin capsules with a white opaque body and a yellow opaque cap. “APO 113” is imprinted on each capsule in black ink; supplied in: Gabapentin Capsules USP, 400 mg are orange/orange size ‘0’ hard gelatin capsules imprinted with ‘D’ on orange cap and ‘04’ on orange body with black edible ink filled with white to off-white crystalline powder. Generic brands of gabapentin capsules, USP are used for postherpetic nerve pain and for add on therapy for partial onset seizures in patients 3 years and older. Gabapentin can cause life-threatening breathing problems, especially if you already have a breathing disorder or if you use other medicines that can make you drowsy or slow your breathing. Gabapentin Capsules, USP - (gab-ah-PEN-tin) What is the most important information I should know about gabapentin capsules? Do not stop taking gabapentin capsules without first talking to your DOSAGE & ADMINISTRATION DOSAGE AND ADMINISTRATION - Gabapentin capsules, USP are given orally with or without food. Gabapentin capsules, USP should be - swallowed whole with plenty of water. o Take gabapentin capsules with water. If you take too much gabapentin, call your healthcare provider or your local Poison Control Center right away at 1-800-222-1222. What should I avoid while taking gabapentin? thcare provider. Taking gabapentin with alcohol or drugs that cause sleepiness or dizziness may make your sleepiness or Taking gabapentin capsules with alcohol or drugs that cause sleepiness or dizziness may make your sleepiness or dizziness worse. Do not drive, operate heavy machinery, or do other dangerous activities until you know how gabapentin capsules affect you. » Gabapentin Capsules contain not less than 90.0 percent and not more than 110.0 percent of the labeled amount of gabapentin (C 9 H 17 NO 2). Packaging and storage Preserve in well-closed containers. Store at controlled room temperature. USP Reference standards 11 USP Gabapentin RS . USP Gabapentin Related Compound A RS . View the USP Certificate or Product Information Sheet in the table above to view additional product details, including available label text and storage information. Gabapentin is commonly used to treat and prevent seizures in people with epilepsy or to treat nerve pain (postherpetic neuralgia) that can occur after a viral infection called shingles. Gabapentin Capsules DEFINITION Gabapentin Capsules contain NLT 90.0% and NMT 110.0% of the labeled amount of gabapentin (C 9 H 17 NO 2). Gabapentin Capsules package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. Gabapentin capsules, USP are indicated as adjunctive therapy in the treatment of partial seizures with and without secondary generalization in patients over 12 years of age with epilepsy. Gabapentin Capsules USP and Gabapentin Tablets USP (gabapentin) are indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. ON 2.1 Dosage for Postherpetic Neuralgia In adults with postherpetic neuralgia, gabapentin may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3. as 900 mg/day (300 mg three times a day). The dose can subsequently be titrated up as needed for pain relief to a dose o. DESCRIPTION Gabapentin capsules, USP are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin USP. The inactive ingredients for the capsules are calcium carbonate, calcium sulfate dihydrate, glyceryl behenate, and pregelatinized starch. DESCRIPTION Gabapentin Capsules, USP are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin. The inactive ingredients for the capsules are magnesium stearate, pregelatinized starch, starch and talc. The 100 mg capsule shell contains gelatin, sodium lauryl sulfate and titanium dioxide. DEFINITION Gabapentin contains NLT 98.0% and NMT 102.0% of gabapentin (C 9 H 17 NO 2), calculated on the anhydrous basis.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |