Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
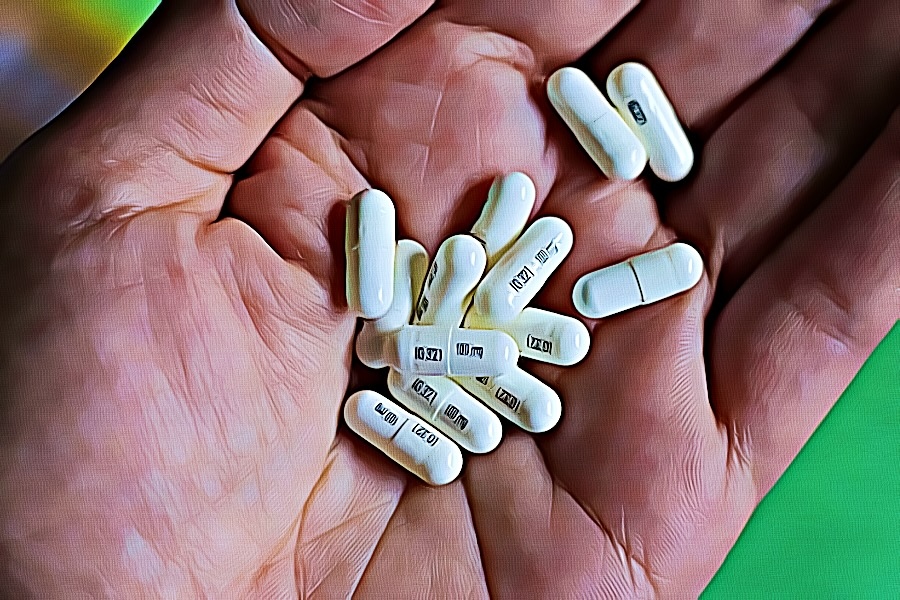
• The Home Office reclassified the prescription drugs pregabalin and gabapentin as class C drugs in April 2019 after concerns about misuse and addiction; • The change in law made it illegal to possess the drugs without a prescription, and supply or sell them to others; • Researchers at Keele University say the immediate impact of the reclassification on prescribing was 'limited', but Understanding how gabapentin is classified within the state is crucial for navigating both medical and legal landscapes. This discussion examines the classification of gabapentin in North Carolina, the legal implications and penalties associated with its use, and recent legislative changes affecting its regulation. In accordance with a new state law, the anticonvulsant and nerve pain medication gabapentin will soon be added to the list of drugs tracked through the state’s prescription drug management program (PDMP), the NC Controlled Substances Reporting System (NC CSRS). While gabapentin remains a non-controlled substance, Session Law 2023-65 Part XI Section 11.1 G.S. 90-113.73 (b) adds it to the Gabapentin is a controlled substance in states like Michigan and Kentucky, while others have mandated reporting rules. Learn about its risk for abuse here. Gabapentin is an anti-epileptic drug, also called an anticonvulsant. It is used to treat some types of seizures and nerve pain caused by shingles. From 1 April 2019 pregabalin and gabapentin will be reclassified as class C controlled substances in the UK. The change, announced in October 2018, is expected to prompt a decline in the use of the drugs as prescribing, dispensing, and collecting them becomes more onerous for doctors, pharmacists, and patients. The reclassification will make it illegal to supply pregabalin and gabapentin Drugs in class C are associated with the least amount of harm compared with those in classes A or B. Pregabalin and gabapentin are currently used to treat conditions like epilepsy, nerve pain and anxiety. The Home Office has said that these medicines will still be available for legitimate use on prescription by a doctor after the change in law. We compared two interventions including (1) class V scheduling of gabapentin and (2) gabapentin inclusion in the state PDMP using a control group of similar states. The Schedule V change for gabapentin was implemented in three states including Kentucky, Tennessee, and West Virginia (Table 1). The change means it will be illegal to possess pregabalin and gabapentin without a prescription and it will be illegal to supply or sell them to others. Briefing Note From 1 April 2019, gabapentin and pregabalin will be reclassified as Schedule 3 controlled drugs under the Misuse of Drugs Regulations 2001, and Class C of the Misuse of Drugs Act 1971. We would like to show you a description here but the site won’t allow us. Gabapentin | Deranged PhysiologyGabapentin Having originally been developed as an anti-seizure medication, gabapentinoids include gabapentin and pregabalin, which are now prescribed primarily for neuropathic pain, seizures and anxiety, but also for fibromyalgia, restless legs syndrome and complications of MS (Chan et al, 2023). First introduced in the UK and the US in 1993, the number of doses of pregabalin and gabapentin taken daily Gabapentin is a prescription medication approved by the FDA for the treatment of neuropathic pain (postherpetic neuralgia) and seizure disorders. Why is gabapentin controlled in some states? Gabapentin is structurally and pharmacologically related to pregabalin (Lyrica, Lyrica CR), which is a Schedule V drug and controlled federally in all states. View gabapentin information, including dose, uses, side-effects, renal impairment, pregnancy, breast feeding, monitoring requirements and important safety information. Only practitioners with a Utah Controlled Substance license and a DEA registration may issue prescriptions for Gabapentin or order the direct administration or dispensing of Gabapentin to a patient. Pregabalin and gabapentin are to be reclassified as Class C controlled substances from April 2019, the government has announced. Structure of GABA: gabapentin and pregabalin. 10 Pharmacokinetics The actions of gabapentinoids are mainly at an intracellular site and require active uptake. They undergo facilitated transport across cell membranes through system l -amino acid transporters (LAT) as both drugs are structurally similar to the amino acid leucine. The effects of chronic gabapentin are blocked by an inhibitor of Gabapentin tablets can be taken with or without food. If you take an antacid containing aluminum and magnesium, such as Maalox®*, Mylanta®*, Gelusil®*, Gaviscon®*, or Di-Gel®*, you should wait at least 2 hours before taking your next dose of gabapentin. The government’s reclassification was expected to reduce prescribing of gabapentinoids, but Elisabeth Mahase finds that a lack of support for GPs and patients is limiting its impact In October 2018, the UK government announced that it would be reclassifying gabapentin and pregabalin1—known collectively as gabapentinoids—after experts pointed out the rising numbers of deaths linked to the
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |