Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
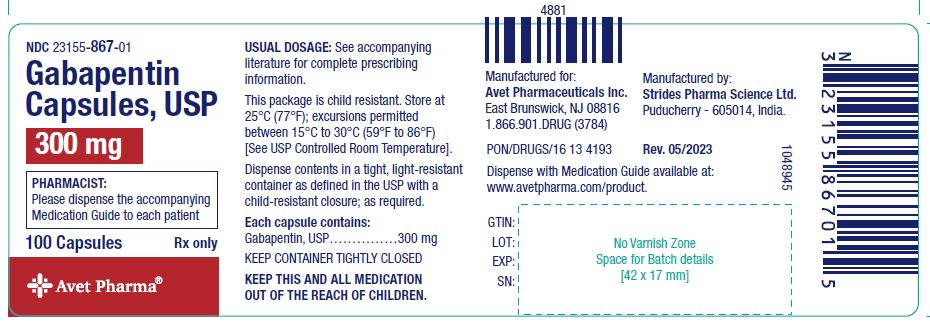 |  |
Gabapentin is chemically known as 2-[1-(aminomethyl) cyclohexaneacetic acid]. Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. Gabapentin is not classified as a controlled substance at a Federal level, however some states in the U.S. have classified gabapentin as a controlled substance at a state level. The states that have classified gabapentin as a controlled substance are Kentucky, Virginia, West Virginia, Michigan, and Tennessee. Regional Variation Gabapentin’s regulatory status varies by state. Some states classify it as a Schedule V controlled substance due to concerns about misuse and its involvement in the opioid crisis. Others do not schedule it but require mandatory reporting to state prescription drug monitoring programs (PDMPs) to track prescribing and dispensing. Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food and Drug Gabapentin is not a federally-controlled drug substance and does not contain an opioid (narcotic) medication. However, gabapentin misuse and abuse has been reported, and it may be restricted in some states through their state drug-monitoring program. Gabapentin is a Schedule V drug in states where it’s classified as a controlled substance. Despite its increasing use, especially for off-label purposes, gabapentin typically does not have the same potential for misuse or dependence as some other drugs, such as opioids or benzodiazepines. At the national level, gabapentin is not classified as a controlled substance under the Controlled Substances Act (CSA). This means it is not subject to the stringent regulations that apply to opioids or benzodiazepines, which are categorized based on their potential for abuse, medical use, and safety. From 1 April 2019 pregabalin and gabapentin will be reclassified as class C controlled substances in the UK. The change, announced in October 2018, is expected to prompt a decline in the use of the drugs as prescribing, dispensing, and collecting them becomes more onerous for doctors, pharmacists, and patients. The reclassification will make it illegal to supply pregabalin and gabapentin Prescription drugs pregabalin and gabapentin are to be reclassified as class C controlled substances from next April, the government announced today (15 October). Today’s move comes after States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. Gabapentin, sold under the brand name Neurontin among others, is an anticonvulsant medication primarily used to treat neuropathic pain and also for partial seizures [10][7] of epilepsy. It is a commonly used medication for the treatment of neuropathic pain caused by diabetic neuropathy, postherpetic neuralgia, and central pain. [11] It is moderately effective: about 30–40% of those given Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Gabapentin and pregabalin – controlled drugs demystified Gabapentin and pregabalin were reclassified as controlled drugs (CDs) back in April 2019, yet many health and social care providers still seem unsure how to treat them. Are they controlled drugs? In short, yes they are controlled drugs but no, they do not need to be locked in a CD cabinet, recorded in a CD register or given with a Gabapentinoids, also known as α2δ ligands, are a class of drugs that are chemically derivatives of the inhibitory neurotransmitter gamma-Aminobutyric acid (GABA) (i.e., GABA analogues) which bind selectively to the α 2 δ protein that was first described as an auxiliary subunit of voltage-gated calcium channels (VGCCs). [1][2][3][4][5] Clinically used gabapentinoids include gabapentin Gabapentin is approved to prevent and control partial seizures, relieve postherpetic neuralgia after shingles and moderate-to-severe restless legs syndrome. Learn what side effects to watch for, drugs to avoid while taking gabapentin, how to take gabapentin and other important questions and answers. Gabapentin is available in both branded and generic forms. From midnight on 1st April 2019, gabapentin and pregabalin will be reclassified as Schedule 3 controlled drugs, under the Misuse of Drugs Regulations (2001), and Class C of the Misuse of Drugs Act (1971), as is already the case with Tramadol. Though the U.S. Drug Enforcement Agency has not reclassified gabapentin, 7 state boards of pharmacy are now acknowledging gabapentin’s abuse potential and have reclassified gabapentin as a Schedule V controlled substance. Gabapentin isn’t a controlled substance according to the federal government. But several states have passed their own laws classifying gabapentin a schedule V (schedule 5) controlled substance. Gabapentin is classified as a controlled substance in several states, including Alabama, Georgia, Kentucky, Tennessee, and Texas. These states have placed it under Schedule V, indicating a lower potential for abuse compared to higher schedules.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |