Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
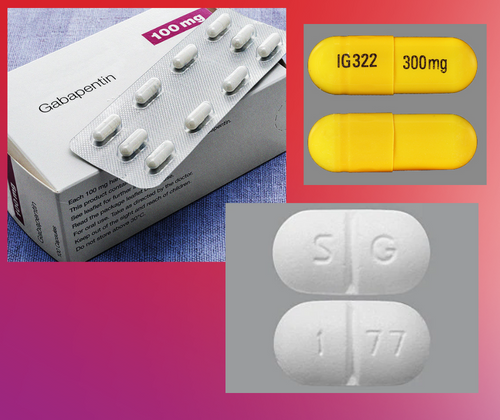
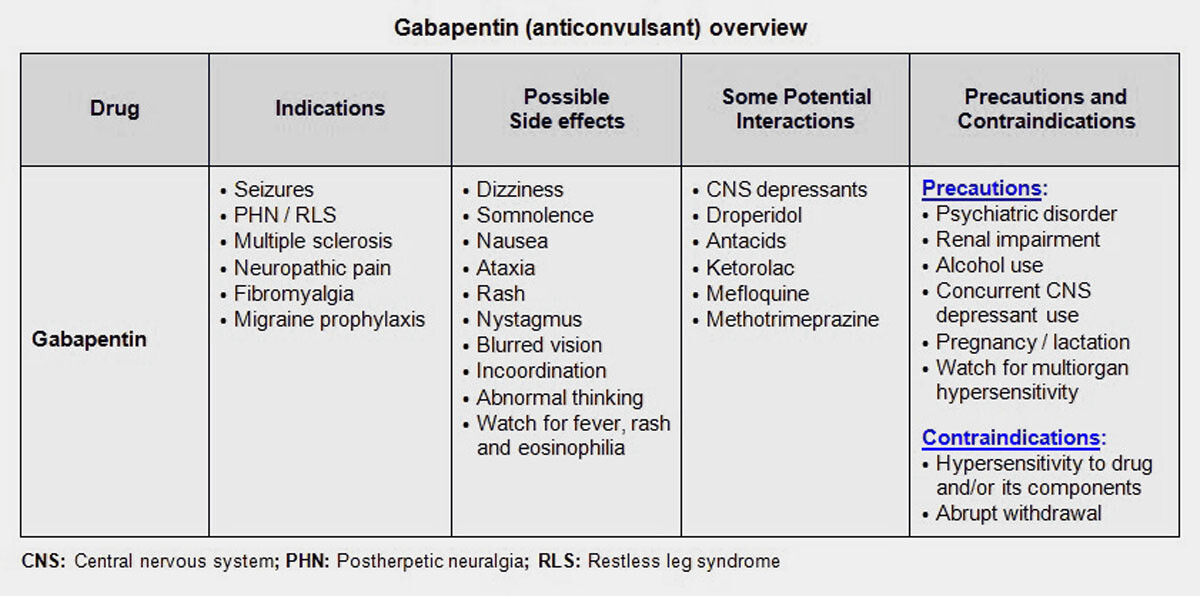
RX DRUG SCHEDULING & MONITORING Prescription Drug Monitoring Programs (PDMPs) are electronic databases that collect information on the dispensing and prescribing of drugs within jurisdictions. PDMPs aim to assist patients in their quality of care by allowing prescribers and dispensers access to the patient’s controlled substance prescription medication history. This access to individual Key takeaways: Gabapentin (Neurontin) is FDA-approved to treat specific types of nerve pain and seizures. It’s also sometimes used to treat other health conditions. These include restless leg syndrome, anxiety, and alcohol withdrawal. Gabapentin isn’t a controlled substance according to the federal government. But several states have passed their own laws classifying gabapentin a schedule Controlled substances are regulated more stringently and monitored using states specific monitoring databases called ‘prescription drug monitoring programs’, or PMPs. In seven states, gabapentin is classified as a schedule V controlled substance (including AL, KY, MI, ND, TN, VA, and WV). Gabapentin is a prescription medication approved by the FDA for the treatment of neuropathic pain (postherpetic neuralgia) and seizure disorders. Why is gabapentin controlled in some states? Gabapentin is structurally and pharmacologically related to pregabalin (Lyrica, Lyrica CR), which is a Schedule V drug and controlled federally in all states. §964. Composition of schedules Schedules I, II, III, IV, and V shall, unless and until added pursuant to R.S. 40:962, consist of the following drugs or other substances, by whatever official name, common or usual name, chemical name, or brand name designated: SCHEDULE I A. Opiates. Unless specifically excepted or unless listed in another schedule, any of the following opiates, including their However, some states have implemented their laws to reclassify gabapentin as a Schedule V controlled substance. In states that label gabapentin as a controlled substance, there may be regulations mandating specific requirements for prescriptions, as well as limits on the quantity prescribed or refills available. Individuals at the highest risk for abusing gabapentin include those with opioid abuse, mental illness, or previous history of prescription drug abuse. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. Gabapentin – or Neurontin – is a medication commonly used to treat nerve pain and seizures. However, the drug can have potentially harmful effects when combined with other opioids. Michigan joins a growing number of states that have scheduled Gabapentin as a controlled substance. Drug Schedules Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules depending upon the drug’s acceptable medical use and the drug’s abuse or dependency potential. The abuse rate is a determinate factor in the scheduling of the drug; for example, Schedule I drugs have a high potential for abuse and the potential to create Gabapentin is classified as a controlled substance in several states, including Alabama, Georgia, Kentucky, Tennessee, and Texas. These states have placed it under Schedule V, indicating a lower potential for abuse compared to higher schedules. In Pennsylvania, the regulation of Gabapentin has become an important issue as officials attempt to control its prescribed and non-medical usage. In 2019, Pennsylvania passed new regulations that categorized Gabapentin as a Schedule V controlled substance. This change was made due to concerns about the drug’s misuse potential. Regional Variation Gabapentin’s regulatory status varies by state. Some states classify it as a Schedule V controlled substance due to concerns about misuse and its involvement in the opioid crisis. Others do not schedule it but require mandatory reporting to state prescription drug monitoring programs (PDMPs) to track prescribing and dispensing. II: N Adzenys ER/XR-ODT, Dyanavel XR, Evekeo, Evekeo ODT Anileridine 9020 II Y Leritine Benzhydrocodone combination products 9193 II Y Apadaz Bezitramide 9800 II Y Burgodin Carfen Pharmacies licensed and located in Minnesota must report to the MN PMP all schedule II-V controlled substance prescriptions, along with prescriptions for butalbital and gabapentin, when dispensed. Individuals at the highest risk for abusing gabapentin include those with opioid abuse, mental illness, or previous history of prescription drug abuse. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. Gabapentin is a Schedule V drug in states where it’s classified as a controlled substance. Despite its increasing use, especially for off-label purposes, gabapentin typically does not have the same potential for misuse or dependence as some other drugs, such as opioids or benzodiazepines. Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of gamma-aminobutyric acid (GABA). Gabapentin isn't a narcotic, but it is a controlled substance in some states. Here's what you should know before using it. See Epilepsy. MHRA/CHM advice: Gabapentin (Neurontin ®) and risk of abuse and dependence: new scheduling requirements from 1 April (April 2019) Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. We would like to show you a description here but the site won’t allow us.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |