Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 | |
 |  |
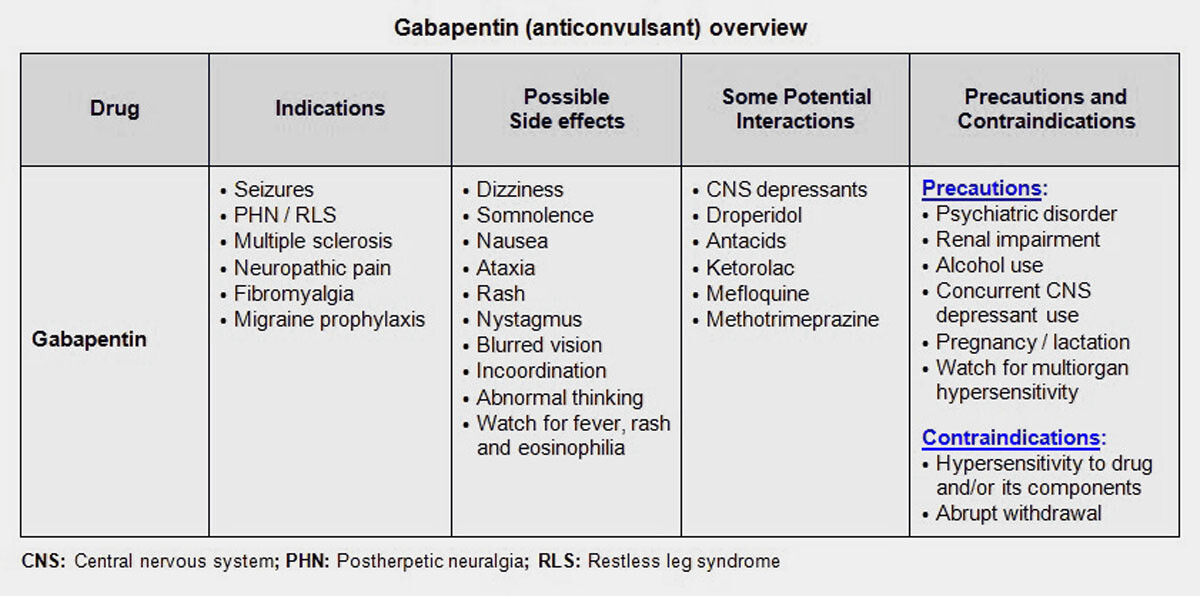
Gabapentin is a prescription medication approved by the FDA for the treatment of neuropathic pain (postherpetic neuralgia) and seizure disorders. Why is gabapentin controlled in some states? Gabapentin is structurally and pharmacologically related to pregabalin (Lyrica, Lyrica CR), which is a Schedule V drug and controlled federally in all states. The number of states placing gabapentin on the controlled substance list or in their required monitoring program is growing and three more states are debating whether to add gabapentin as a controlled substance or to their mandated reporting programs (DE, NY, and WI). Purpose Requires the Arizona State Board of Pharmacy (Board) to adopt the Schedule I, II, III, IV and V controlled substance designations as listed in federal law. Gabapentin is a controlled substance in states like Michigan and Kentucky, while others have mandated reporting rules. Learn about its risk for abuse here. Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of gamma-aminobutyric acid (GABA). Gabapentin isn't a narcotic, but it is a controlled substance in some states. Here's what you should know before using it. Arizona Prescription Monitoring Program The Arizona Prescription Monitoring Program (PMP), housed in the Arizona State Board of Pharmacy, collects data on all controlled substance prescriptions in Arizona (schedules II-V). This information assists healthcare providers in making better-informed care decisions when treating patients. Gabapentin, originally developed to treat epilepsy, has gained popularity as a medication for neuropathic pain and other conditions. However, its increasing use has raised concerns about potential misuse and addiction. As a result, various states have begun to classify gabapentin as a controlled substance. Understanding the legal status of gabapentin across different jurisdictions is crucial We would like to show you a description here but the site won’t allow us. Learn whether gabapentin is a controlled substance, as well as risks of prescription drug abuse and treatment for gabapentin addiction. Gabapentin isn’t classified as a controlled substance under federal law in the United States. But it is classified as a controlled substance in some states. Abstract The abuse potential of gabapentin is well documented; with gabapentin having been noted as an agent highly sought after for use in potentiating opioids. When combined with opioids, the risk of respiratory depression and opioid-related mortality increases significantly. In the US, gabapentin was approved by the Food and Drug Administration as a non-controlled substance. To date, and in The positivity rate for gabapentin was 26% among drug overdose decedents who tested positive for opioids.i In 2019, the FDA announced a new mandate that labels of gabapentin and pregabalin contain a warning about respiratory depression.ii Pregabalin is controlled in Schedule V of the Federal Controlled Substances Act. Here's who gabapentin was originally approved for, what it's used for today and why it's becoming a drug of increasing concern for abuse and misuse. However, some states have implemented their laws to reclassify gabapentin as a Schedule V controlled substance. In states that label gabapentin as a controlled substance, there may be regulations mandating specific requirements for prescriptions, as well as limits on the quantity prescribed or refills available. Regional Variation Gabapentin’s regulatory status varies by state. Some states classify it as a Schedule V controlled substance due to concerns about misuse and its involvement in the opioid crisis. Others do not schedule it but require mandatory reporting to state prescription drug monitoring programs (PDMPs) to track prescribing and dispensing. The State of Arizona requires outpatient dispensing pharmacies and providers who dispense out of their office to report controlled substance II-V dispensations to the PMP through the Clearinghouse program. Pursuant to A.R.S. 36-2608, every outpatient pharmacy with an active DEA number and a valid Arizona pharmacy permit, that is not limited to veterinary dispensing, must have a Clearinghouse As healthcare professionals, Pharmacists play a very critical role in helping Arizona solve the prescription drug misuse and abuse problem in our state. These guidelines are intended to help Dispensers reduce the inappropriate use of controlled substances while preserving the vital role of the Pharmacy to treat patients with medical conditions. These guidelines were developed at the Arizona Alcohol & Drug Trends States Consider Listing Gabapentin as Controlled Substance (Spring 2018) Another drug is rising through the ranks when it comes to abuse: gabapentin. It has been prescribed for over two decades to combat epilepsy and relieve nerve pain (and off-label for anxiety, bipolar disorder and migraines). Now its illegal use is Arizona Controlled Substances Prescription Monitoring Program Electronic Prescribing of Controlled Substances (EPCS) Pharmacy Requirements Beginning January 1, 2020, a schedule II controlled substance that is an opioid may be dispensed only with an electronic prescription order as prescribed by federal law or regulation.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 | |
 |  |