Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
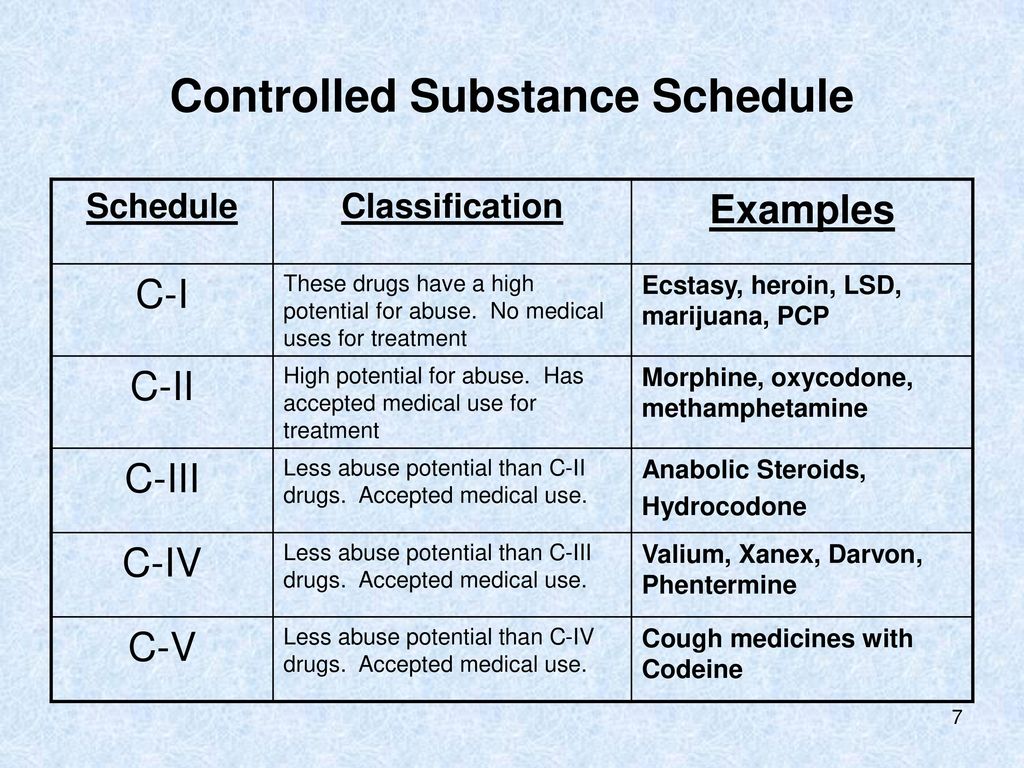
Gabapentin is a prescription medication approved by the FDA for the treatment of neuropathic pain (postherpetic neuralgia) and seizure disorders. Why is gabapentin controlled in some states? Gabapentin is structurally and pharmacologically related to pregabalin (Lyrica, Lyrica CR), which is a Schedule V drug and controlled federally in all states. PDF RCW 69.50.212 Schedule V. Unless specifically excepted by state or federal law or regulation or more specifically included in another schedule, the following controlled substances are listed in Schedule V: Individuals at the highest risk for abusing gabapentin include those with opioid abuse, mental illness, or previous history of prescription drug abuse. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. The controlled substances listed in this section may be added, rescheduled, or deleted as provided for in RCW 69.50.201. [ 2013 c 19 s 91; 2010 c 177 s 4; 1993 c 187 s 8; 1986 c 124 s 5; 1980 c 138 s 3; 1971 ex.s. c 308 s 69.50.208.] (a) [ (1)] A controlled substance may be dispensed only as provided in this section. Prescriptions electronically communicated must also meet the requirements under RCW 69.50.312. About the PDMP The Prescription Drug Monitoring Program (PDMP) aims to improve the District’s ability to identify and reduce diversion of prescription drugs in an efficient and cost effective manner that will not impede the appropriate medical utilization of controlled substances. The PDMP also aims to enhance patient care by providing prescription monitoring information that will assure Gabapentin isn't a narcotic, but it is a controlled substance in some states. Here's what you should know before using it. Abstract The abuse potential of gabapentin is well documented; with gabapentin having been noted as an agent highly sought after for use in potentiating opioids. When combined with opioids, the risk of respiratory depression and opioid-related mortality increases significantly. In the US, gabapentin was approved by the Food and Drug Administration as a non-controlled substance. To date, and in Gabapentin isn’t classified as a controlled substance under federal law in the United States. But it is classified as a controlled substance in some states. RCW 70.225 (2007) created Washington's PMP also known as Prescription Monitoring Program. The program was created to improve patient care and to stop prescription drug misuse by collecting dispensing records for Schedule II, III, IV and V drugs, and by making the information available to medical providers and pharmacists as a patient care tool. Gabapentin can be used to treat a variety of conditions, but in some cases it has the potential for abuse. Currently, however, the Drug Enforcement Administration (DEA) has not classified gabapentin as a controlled substance per federal law. Evidence suggests that gabapentin abuse and addiction in Washington state is on the rise. Avoid alcohol use during gabapentin treatment. Avoid other central nervous system depressants, including drugs that make you sleepy or dizzy. Future of gabapentin regulation As of June 2024, gabapentin remains a non-controlled substance under the U.S. federal government. Individuals at the highest risk for abusing gabapentin include those with opioid abuse, mental illness, or previous history of prescription drug abuse. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. New Prescribing Rules Information for Providers New opioid prescribing requirements are coming for many practitioners in Washington State. The goals are to increase public health and safety by reducing opioid abuse and misuse, while ensuring that practitioners continue to treat patients safely and appropriately for pain. Gabapentin, initially developed for epilepsy, is now widely used for nerve pain and other off-label applications. Rising prescription rates have sparked discussions about whether it should be classified as a controlled substance due to concerns over misuse and dependency. Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of gamma-aminobutyric acid (GABA). Gabapentin, originally developed to treat epilepsy, has gained popularity as a medication for neuropathic pain and other conditions. However, its increasing use has raised concerns about potential misuse and addiction. As a result, various states have begun to classify gabapentin as a controlled substance. Understanding the legal status of gabapentin across different jurisdictions is crucial Gabapentin is a controlled substance in states like Michigan and Kentucky, while others have mandated reporting rules. Learn about its risk for abuse here. The PDMP is an electronic database used to monitor and collect data on the dispensation of prescription data for Schedule II, III, IV and V controlled substances, as well as products containing the covered substances Butalbital, Cyclobenzaprine and Gabapentin. We would like to show you a description here but the site won’t allow us.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |