Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
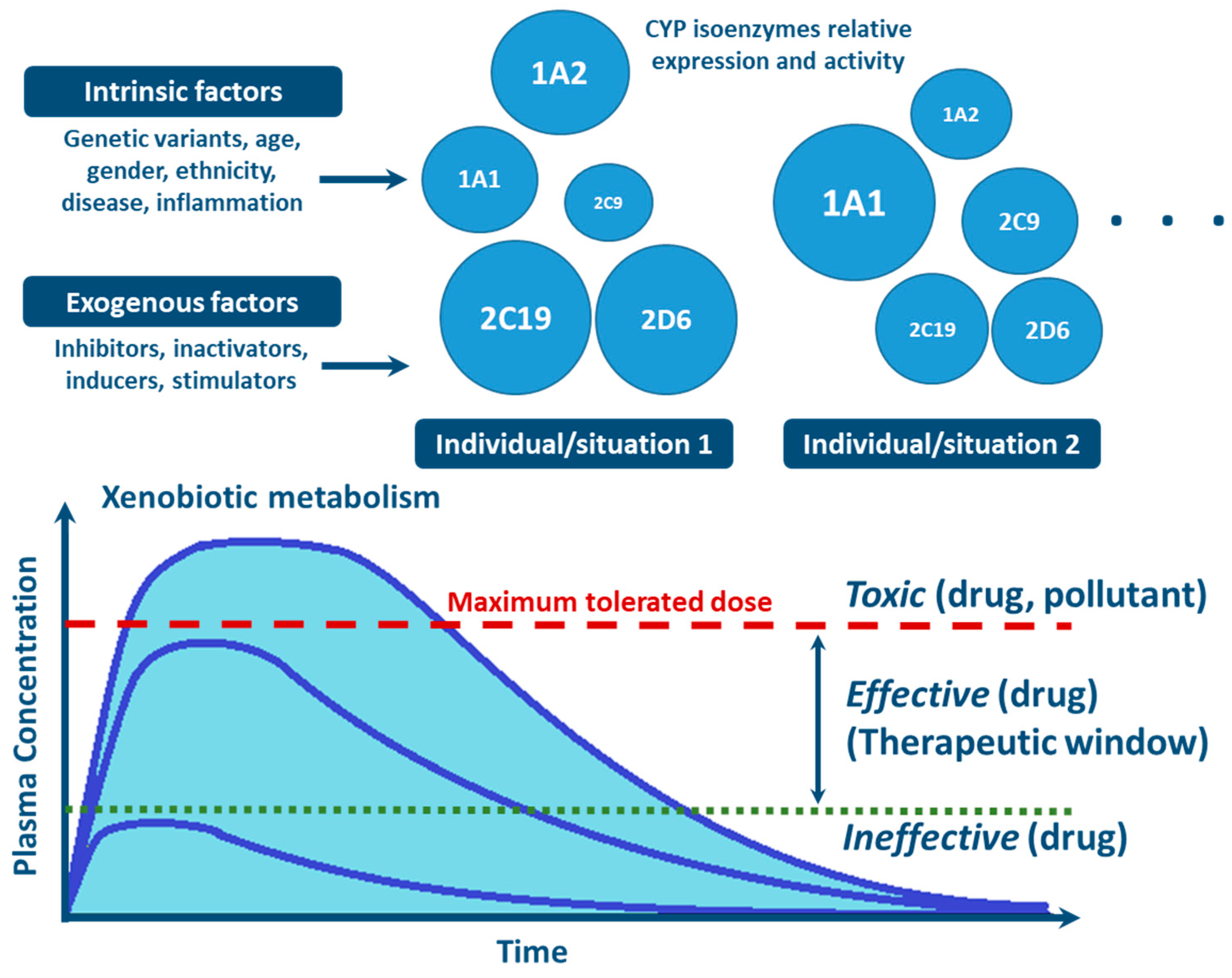
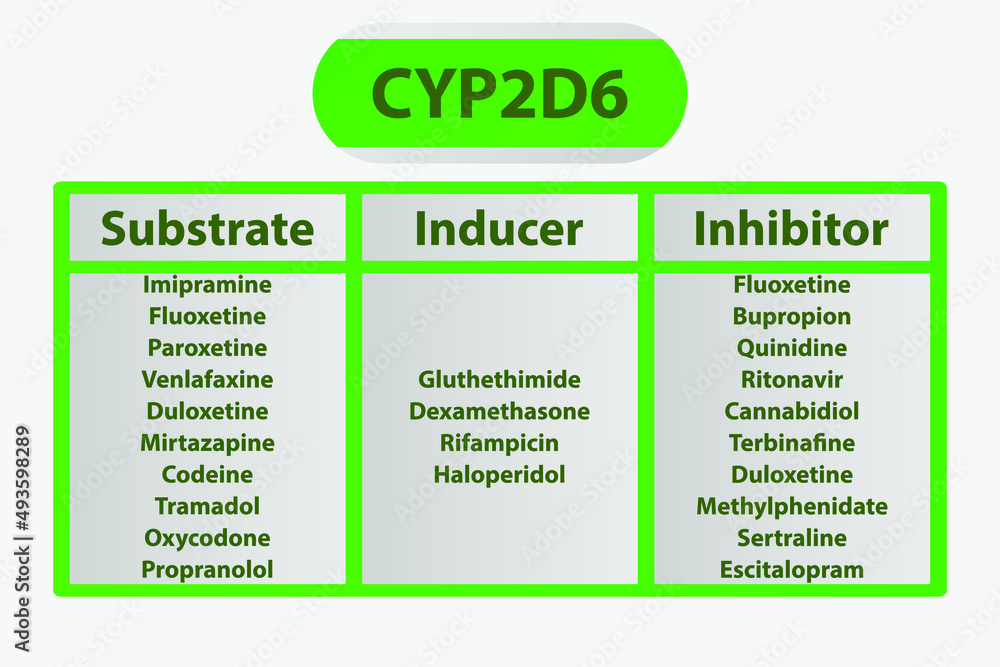
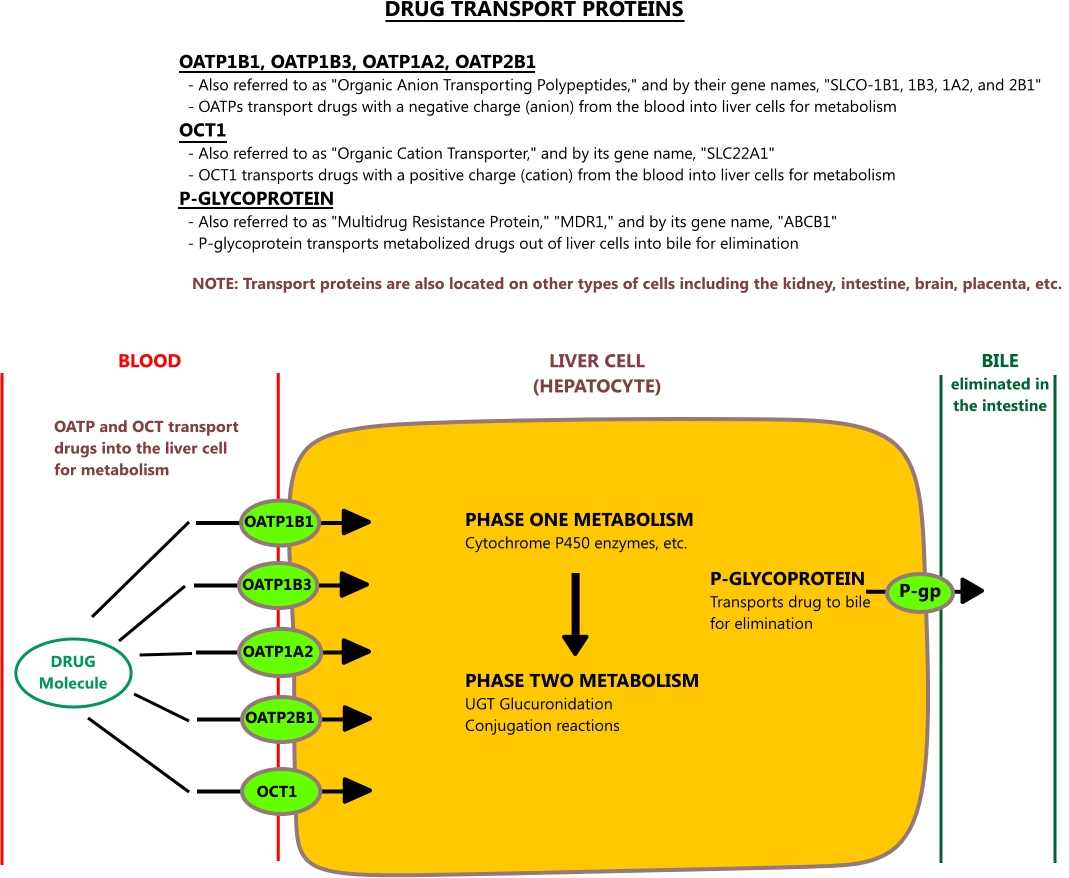
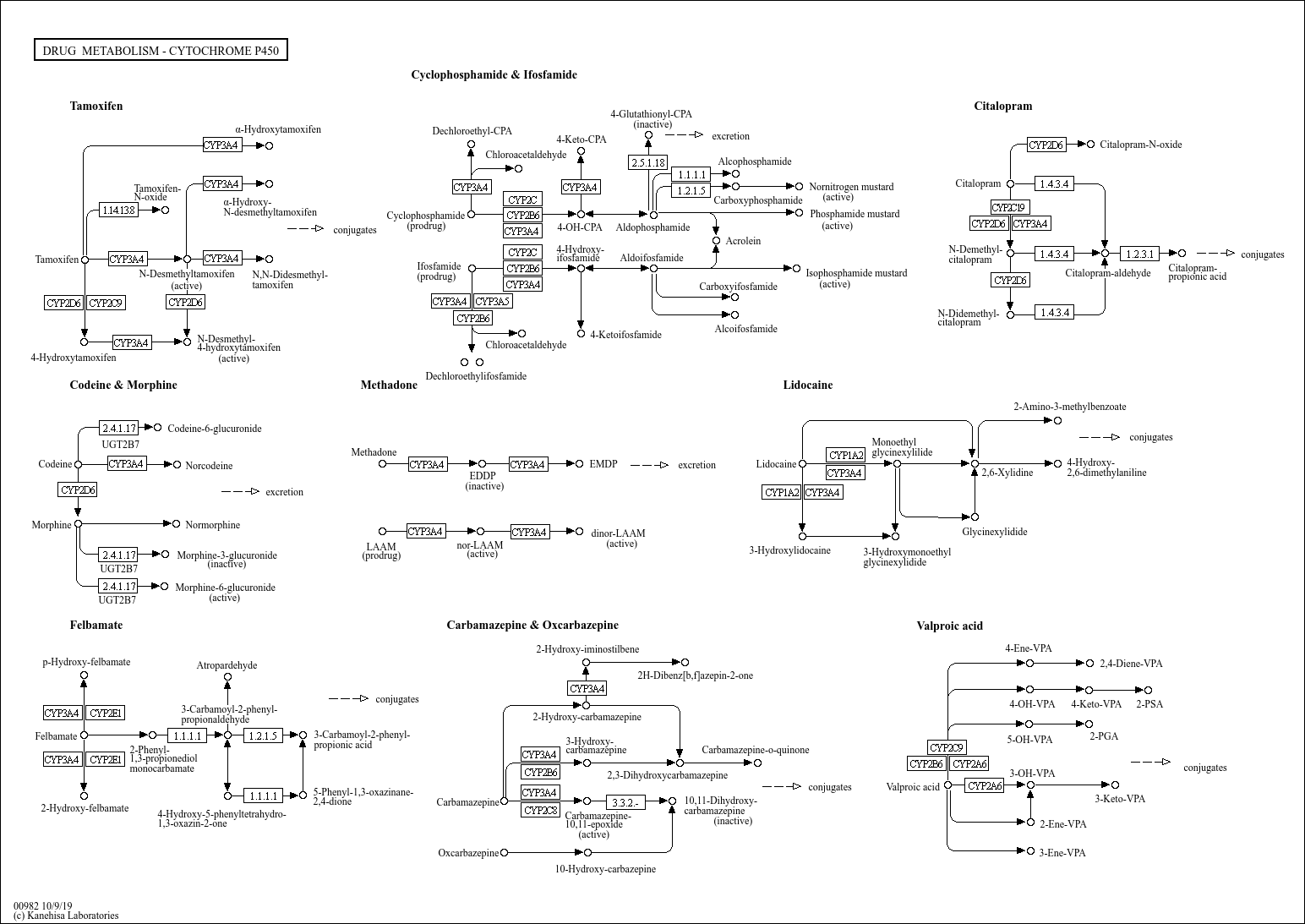
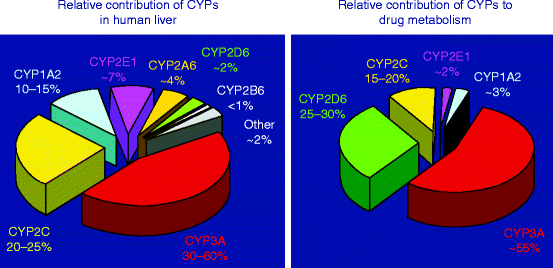
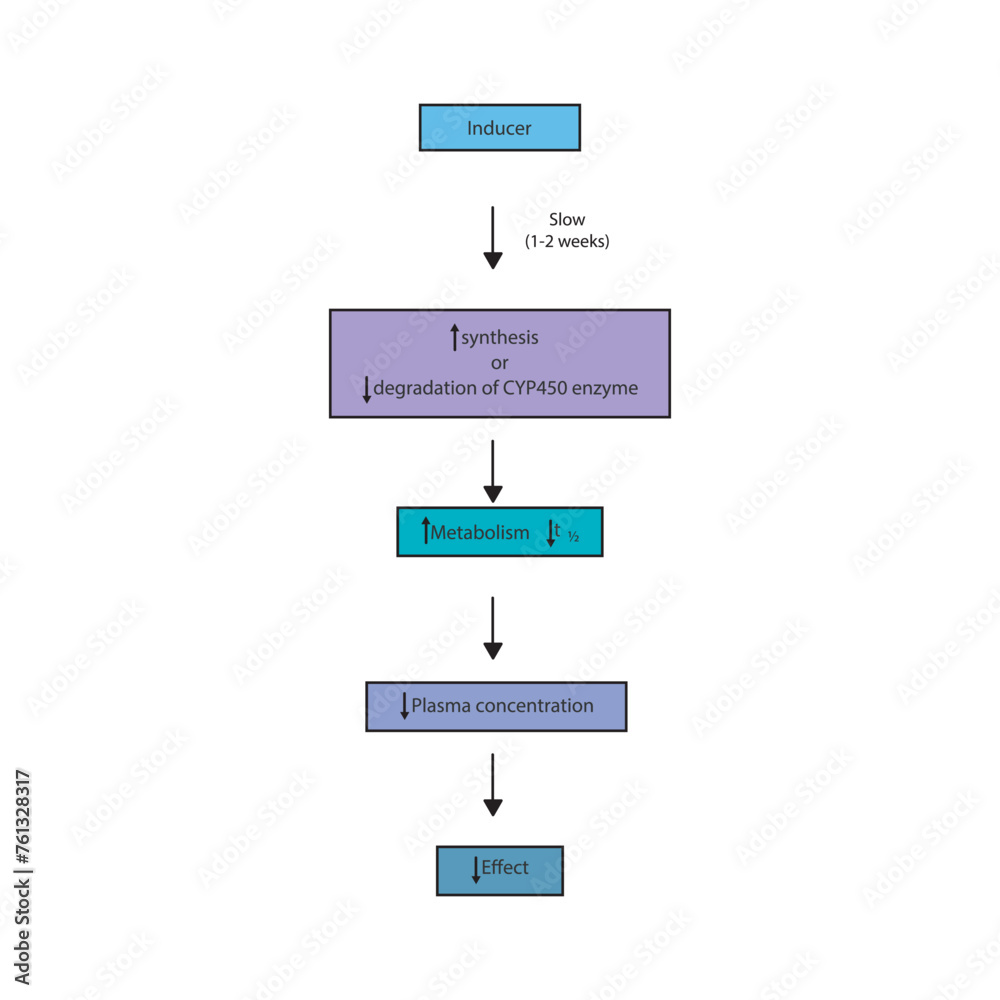
19 CYP Metabolism Insert a row and add your medication, in the same order as the book table of contents. Gabapentin and gabapentin enacarbil are used for management of postherpetic neuralgia (PHN) in adults. Considered one of several first-line therapies for PHN; more effective than placebo, but evidence suggests that only a small proportion of patients will derive a clinically meaningful benefit. The gabapentinoids are often recommended as first-line treatments for the management of neuropathic pain. The differing pharmacodynamic and pharmacokinetic profiles can have implications for In addition, gabapentin does not undergo hepatic metabolism, unlike most other antiepileptic drugs, and is eliminated almost entirely by renal excretion with a clearance that approximates the glomerular filtration rate. Due to a lack of hepatic metabolism, and a low degree of protein binding, gabapentin has very limited potential for drug interactions. In addition, gabapentin does not inhibit or induce the major cytochrome P450 enzymes responsible for drug metabolism. The new antiepileptic medications are prescribed for the treatment of patients with seizure disorders since 17 years ago. Gabapentin (GBP) was approved on January 1994 as adjunctive treatment in patients 12 years or older with partial seizures, with The clinical pharmacokinetics of the 4 antiepileptic drugs lamotrigine, vigabatrin, gabapentin and oxcarbazepine have been reviewed in this paper. All the drugs have linear kinetics and reliable absorption, although the saturation of transport across the gut may occur at high doses with gabapentin. Co-administration of gabapentin, which is not appreciably metabolized by CYP enzymes [47], did not significantly change the concentrations of tamoxifen, NDMT or Z-endoxifen. Gabapentin is not bound to plasma proteins, does not induce hepatic enzymes and is not metabolized. At steady state, it has a half-life of 6-8 h, and is eliminated unchanged by renal route with a plasma clearance proportional to the creatinine clearance. They are not metabolised by the liver and do not affect the cytochrome P450 system, major cytochrome P450 system isoenzymes; however, drug-induced hepatotoxicity has been described in case reports. 16 Elimination is mostly done by the kidney and is proportional to the creatinine clearance. It is an active metabolite with anticonvulsant activity and minor metabolites, including (R)-licarbazepine (4.5%) and oxcarbazepine (0.5%) formed by non-CYP450-mediated metabolism. 1, 52 Eslicarbazepine acetate has a lower risk for drug interactions due to lack of CYP450-dependent metabolism. The gabapentinoids are often recommended as first-line treatments for the management of neuropathic pain. The differing pharmacodynamic and pharmacokinetic profiles can have implications for clinical practice. This article has summarised these key differences. Gabapentin: Cytochrome P450 Metabolism Pharmacodynamics Mechanism of Action Gabapentin is designed as GABA analog (similar to pregabalin), which means it binds to the α2δ (alpha-2-delta) subunit of presynaptic voltage-sensitive Ca2+ channels (VSCCs), and block the release of excitatory neurotransmitters such as glutamate. Metabolism: In humans, gabapentin undergoes minimal metabolic alteration, largely retaining the original structure. Gabapentin does not induce or inhibit CYP enzymes. As a result, metabolism-related factors do not necessitate dosage alterations of gabapentin and concomitant AEDs after prolonged therapy. The drug is excreted unchanged in urine; plasma clearance is linearly related to creatinine clearance; and dosage is readily adjusted based on renal function. A cytochrome P450 monooxygenase involved in the metabolism of various endogenous substrates, including fatty acids, steroid hormones and vitamins (PubMed:11093772, PubMed:14559847, PubMed:15766564, PubMed:19965576, PubMed:7574697). In vitro studies were conducted to investigate the potential of gabapentin to inhibit the major cytochrome P450 enzymes (CYP1A2, CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4) that mediate drug and xenobiotic metabolism using isoform selective marker substrates and human liver microsomal preparations. The aim of this paper is to review a number of new antiepileptic agents (i.e. felbamate, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, tiagabine, topiramate, vigabatrin and zonisamide) for their inducing and/or inhibitory properties in humans, mainly considering the interactions where they The pharmacokinetic characteristics of gabapentin are described, and the interaction effects of concomitant antiseizure medications on gabapentin in terms of its pharmacokinetics and pharmacodynamics are highlighted. Ethosuximide, gabapentin, tiagabine, and vigabatrin are neither inducers nor inhibitors of drug metabolism. Hepatic enzyme inhibition usually occurs because of competition at the enzyme site. Knowledge of the specific metabolic enzymes involved in the metabolism of AEDs allows clinicians to predict potential interactions.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |