Gallery
Photos from events, contest for the best costume, videos from master classes.
 | /cloudfront-us-east-1.images.arcpublishing.com/gray/4JJR2WORPBDQ7GBFZVQCBO3C5Q.png) |
 | /cloudfront-us-east-1.images.arcpublishing.com/gray/WYT5UICGFJHHBI4TTNZKNFFS74.png) |
 |  |
 |  |
 |  |
 | 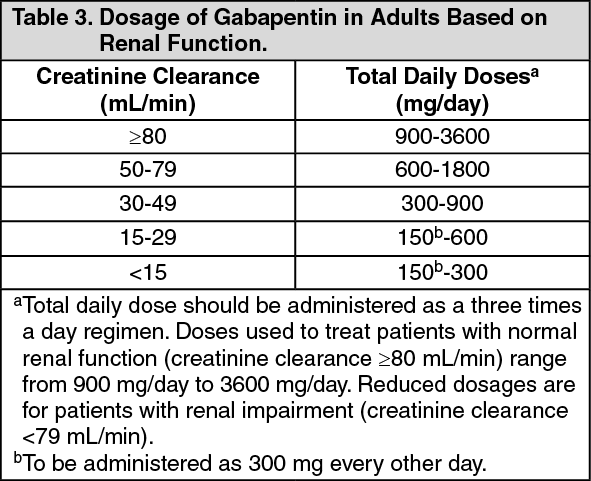 |
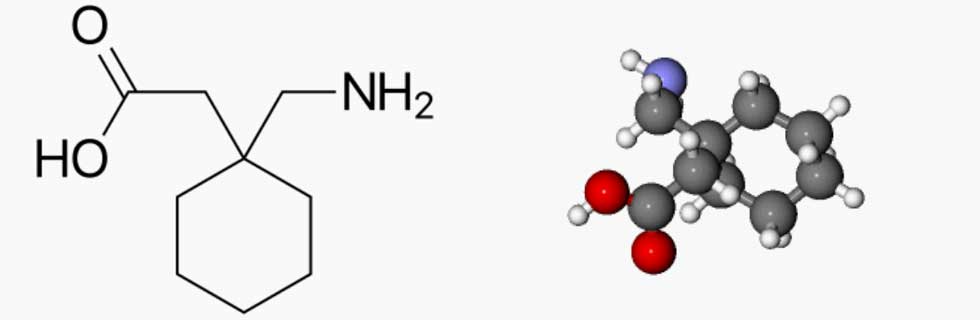
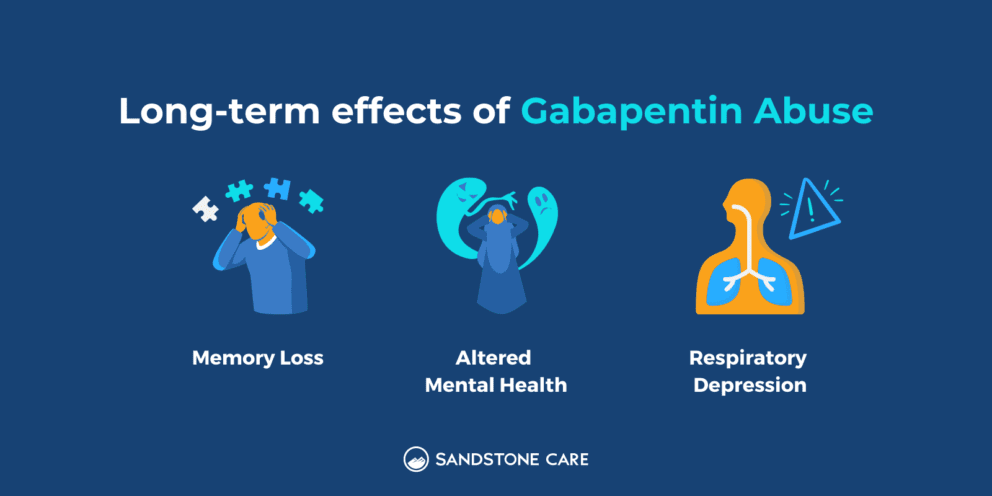
Since its market release, gabapentin has been presumed to have no abuse potential and subsequently has been prescribed widely off-label, despite increasing reports of gabapentin misuse. This review estimates and describes the prevalence and effects Gabapentin, a Schedule V drug in the Commonwealth, had previously been considered a “drug of concern” in Virginia. The 2019 Virginia General Assembly passed HB2557, which classified gabapentin as a Schedule V controlled substance as of July 1, 2019. Introduction: Gabapentin is a prescription medication approved by the United States Food and Drug Administration (FDA) for the treatment of neuropathic pain and epileptic disorders. This drug is currently marketed in capsule, tablet, and oral solution formulations. In recent years, however, gabapentin has been increasingly encountered by law enforcement, documented in national crime lab Gabapentin (Neurontin) and pregabalin (Lyrica) are both gabapentinoids—psychotropic medications that cross the blood-brain barrier and mimic the inhibitory neurotransmitter Gamma-aminobutyric acid (GABA). Gabapentin was first approved by the Food and Drug Administration (FDA) in 1993 as an adjunctive treatment for partial seizures. In 2002, the medication was approved for the treatment of The increase is a direct result of the additional reporting requirement of two drugs of concern (promethazine in oral liquid formulations and gabapentin containing products) as of January 20, 2021. There is a continued downward trend in the number of opioid and controlled dangerous substance prescription dispensations to Louisiana patients. Gabapentin’s classification as a “drug of concern” in Indiana has significant implications for healthcare providers. Physicians, pharmacists, and other medical professionals must navigate a complex regulatory environment to ensure compliance with state laws. Currently, gabapentin is classified as a Schedule VI drug of concern. This change in schedule will require gabapentin to be reported to the Virginia Prescription Monitoring Program as a schedule V substance. Gabapentin is a drug of concern, except when dispensed pursuant to a prescription issued by a veterinarian. G. “Patient” means the ultimate user of a drug for whom a prescription is issued and for whom a drug is dispensed. Information on Kansas Administrative Regulations.Agency 68 State Board of Pharmacy Article 21.—Prescription Monitoring Program Gabapentin is an anti-epileptic drug, also called an anticonvulsant. It is used to treat some types of seizures and nerve pain caused by shingles. Gabapentin, originally developed to treat epilepsy, has gained popularity as a medication for neuropathic pain and other conditions. However, its increasing use has raised concerns about potential misuse and addiction. As a result, various states have begun to classify gabapentin as a controlled substance. Understanding the legal status of gabapentin across different jurisdictions is crucial Gabapentin is approved to prevent and control partial seizures, relieve postherpetic neuralgia after shingles and moderate-to-severe restless legs syndrome. Learn what side effects to watch for, drugs to avoid while taking gabapentin, how to take gabapentin and other important questions and answers. Gabapentin is available in both branded and generic forms. This inquiry focused on the regulatory and pharmacovigilance policies of US states and jurisdictions and was primarily conducted via searches of the world-wide-web via the following terms, either alone or in concert: prescription drug monitoring program (PDMP); gabapentin; Schedule-V; controlled substance; “drug of concern”. Moreover, some risks of Gabapentin may lead patients to explore alternative solutions. For instance, learning about the safety concerns around water bodies, like why Lake Erie is so dangerous, can foster a critical perspective about environmental factors affecting health. orizing prescriber or dispenser retains accountability. Drugs of Concern—drugs other than controlled substances as defined by rule whose use requires tracking for public health purposes or which demonstrate a potential for abuse, including any material, compound, mixture, or preparation containing any quantity of the following substances Drugs of Concern Mr. Fontenot reminded the council that during the January 8, 2020 meeting of the PMP Advisory Council they made a recommendation to the Board of Pharmacy to add gabapentin and promethazine containing products to the list of drugs of concern which are identified in regulation. Here's who gabapentin was originally approved for, what it's used for today and why it's becoming a drug of increasing concern for abuse and misuse. Here's who gabapentin was originally approved for, what it's used for today and why it's becoming a drug of increasing concern for abuse and misuse. Gabapentin is an anticonvulsant medication prescribed for a variety of conditions. Learn about its uses, side effects, and what you should know if you've been prescribed this medication. Gabapentin is generally well tolerated and safe for most people to use. But as with any medication, there’s a risk of side effects. Misuse can increase the risk of side effects.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 | /cloudfront-us-east-1.images.arcpublishing.com/gray/4JJR2WORPBDQ7GBFZVQCBO3C5Q.png) |
 | /cloudfront-us-east-1.images.arcpublishing.com/gray/WYT5UICGFJHHBI4TTNZKNFFS74.png) |
 |  |
 |  |
 |  |
 |  |