Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
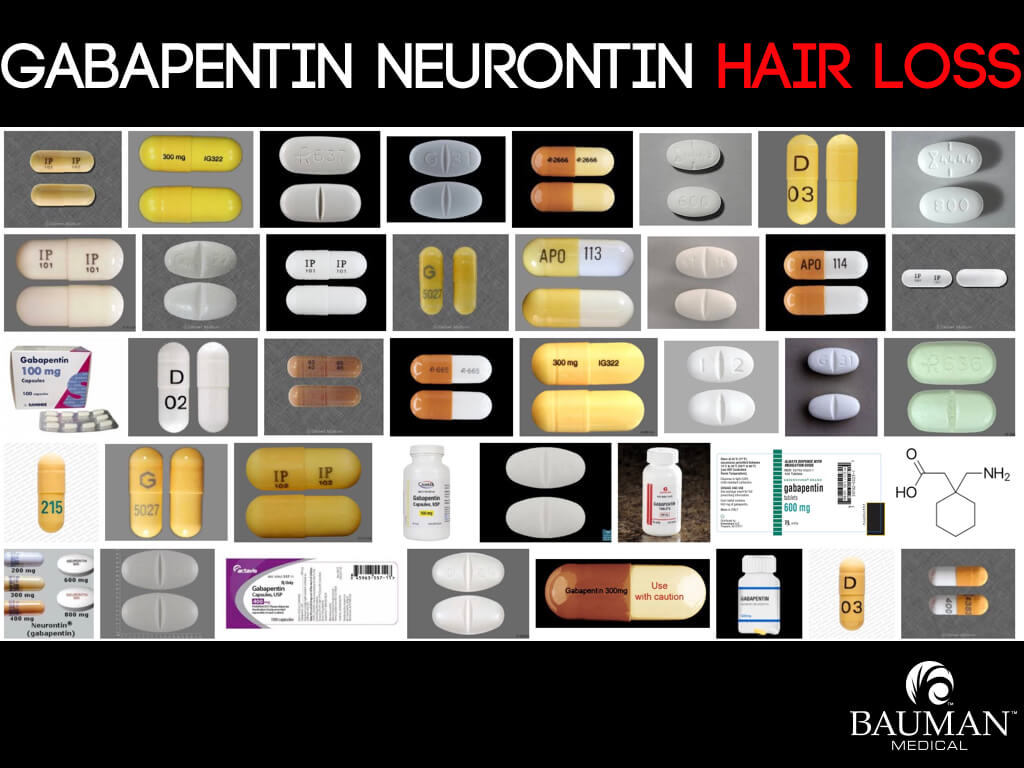
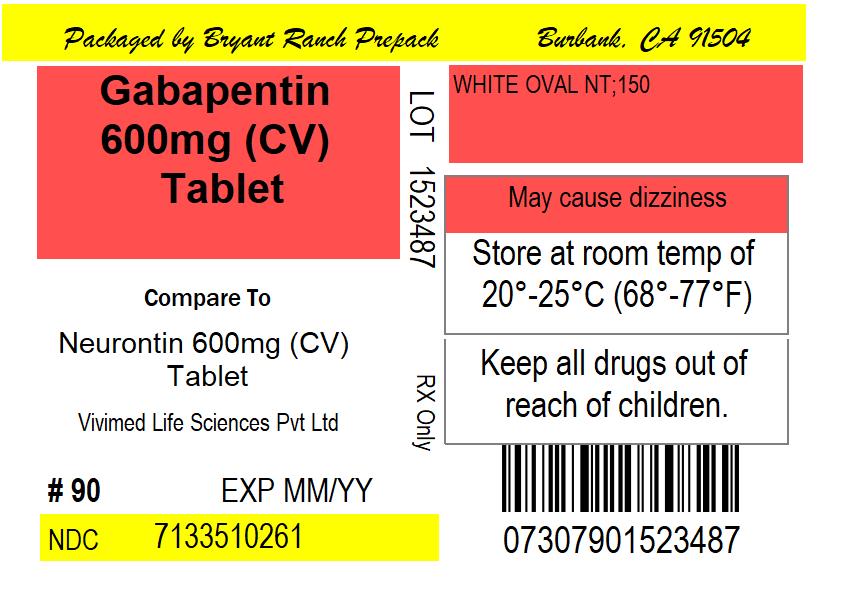
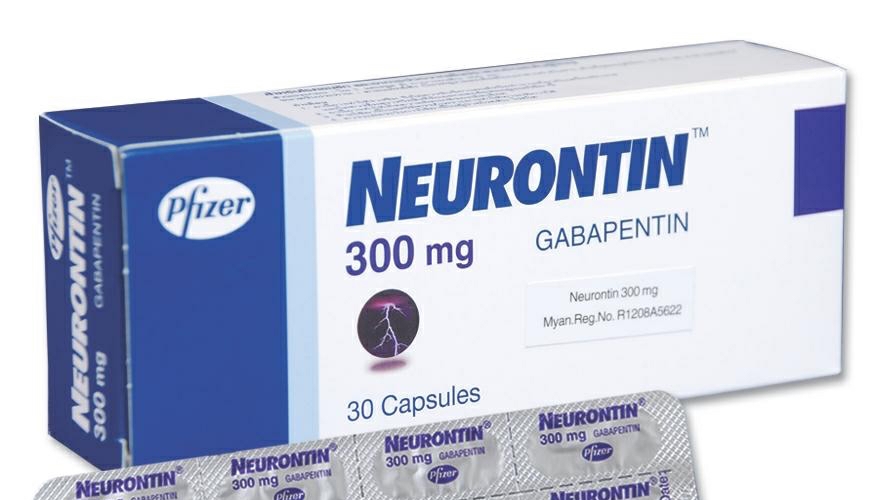
DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. According to the FDA-approved product label, gabapentin is used clinically for the management of postherpetic neuralgia in adults and as an adjunctive therapy for the treatment of partial onset seizures, with and without secondary generalization in adults and pediatric patients 3 years and older with epilepsy. FDA product labeling for this product includes 1 indications and usage, 2.1 dosage for postherpetic neuralgia, 2.2 dosage for epilepsy with partial onset There are rare postmarketing reports of individuals experiencing withdrawal symptoms shortly after discontinuing higher than recommended doses of gabapentin used to treat illnesses for which the drug is not approved. In adults with postherpetic neuralgia, NEURONTIN may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times Neurontin® (gabapentin) is indicated as adjunctive therapy in the treatment of partial seizures with and without secondary generalization in patients over 12 years of age with epilepsy. Gabapentin is widely used in the United States for a number of off-label indications, often as an alternative to opioid therapy. Increasing evidence has emerged suggesting that gabapentin may not be as benign as once thought and may be associated with substance abuse in concert with opioids. Detailed FDA approval information for Gabapentin including labelers, registrants, and establishments In December 1993, the US Food and Drug Administration (FDA) granted approval for gabapentin, under the brand name Neurontin, for adjunctive therapy of partial seizures. Gabapentin and pregabalin are FDA-approved for a variety of uses include fibromyalgia and restless legs syndrome. Gabapentin was first approved in 1993 and pregabalin was Gabapentin is FDA-approved as Neurontin to treat partial seizures in adults and children with epilepsy. Partial seizures are convulsions that originate from a single location in the brain. Neurontin is also approved to treat a type of nerve pain called postherpetic neuralgia, or PHN. DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. Important Limitation: GRALISE is not interchangeable with other gabapentin products because of differing pharmacokinetic profiles that affect the frequency of administration (See Warnings and Precautions) DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. When prescribing NEURONTIN, carefully evaluate patients for a history of drug abuse and observe them for signs and symptoms of gabapentin misuse or abuse (e.g., self-dose escalation and NEURONTIN® (gabapentin) capsules, for oral use NEURONTIN® (gabapentin) tablets, for oral use NEURONTIN® (gabapentin) oral solution Initial U.S. Approval: 1993 FDA is warning that serious breathing difficulties may occur in patients using gabapentin or pregabalin who have respiratory risk factors. Advise the patient to read the FDA-approved patient labeling (Medication Guide). Administration Information - Inform patients that gabapentin capsules are taken orally with or without Most of the individuals described in these reports had a history of poly 329 substance abuse or used gabapentin to relieve symptoms of withdrawal from other substances. 330 When prescribing products that deliver gabapentin, carefully evaluate patients for a 331 history of drug abuse and observe them for signs and symptoms of gabapentin misuse
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |