Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
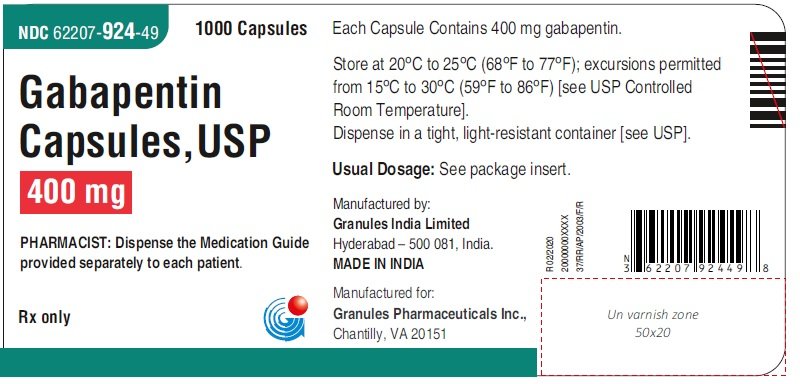
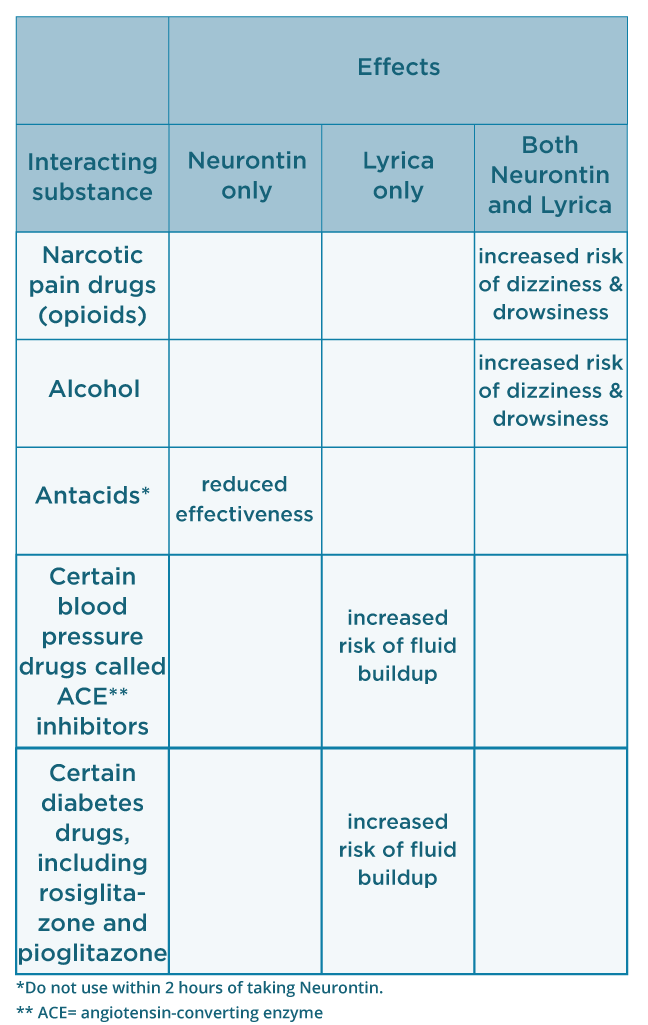
FDA approval history for Horizant (gabapentin enacarbil) used to treat Restless Legs Syndrome, Postherpetic Neuralgia. Supplied by Azurity Pharmaceuticals, Inc. Gabapentin, a drug that can be used in the management of withdrawal symptoms, is a pharmaceutical used in the treatment of addiction. Gabapentin gained FDA approval in 1993 under the brand name Neurontin as an adjunctive therapy in the treatment of partial onset seizures, and subsequently for the treatment of postherpetic neuralgia in adults in 2002. It became available as a generic in 2004. In 2009, the U.S. Food and Drug Administration (FDA) issued a warning of an increased risk of suicidal thoughts and behaviors in patients taking some anticonvulsant drugs, including gabapentin, [75] modifying the packaging inserts to reflect this. [15] A 2010 meta-analysis supported the increased risk of suicide associated with gabapentin use. [76] Gabapentin, sold under the brand name Neurontin among others, is an anticonvulsant medication primarily used to treat neuropathic pain and also for partial seizures [10][7] of epilepsy. It is a commonly used medication for the treatment of neuropathic pain caused by diabetic neuropathy, postherpetic neuralgia, and central pain. [11] It is moderately effective: about 30–40% of those given When prescribing gabapentin carefully evaluate patients for a history of drug abuse and observe them for signs and symptoms of gabapentin misuse or abuse (e.g., development of tolerance, self-dose escalation, and drug-seeking behavior). Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. However, it was later discovered that gabapentin has the potential of an anticonvulsive medication and can be used as an adjunct to more In December 1993, the US Food and Drug Administration (FDA) granted approval for gabapentin, under the brand name Neurontin, for adjunctive therapy of partial seizures. Subsequently, the FDA approved gabapentin in 2000 for treatment of partial seizures in children aged 3 years or older and in 2002 Gabapentin is widely used in the United States for a number of off-label indications, often as an alternative to opioid therapy. Increasing evidence has emerged suggesting that gabapentin may not be as benign as once thought and may be associated DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. Neurontin® and generic gabapentin immediate-release dosing is FDA-approved for use as adjunctive therapy for partial seizures with or without generalization in pediatric epileptic patients 12 years of age and older, as well as adjunctive therapy for partial seizures in pediatric patients 3 years to 11 years of age.1-3, Gabapentin extended These highlights do not include all the information needed to use NEURONTIN safely and effectively. See full prescribing information for NEURONTIN. NEURONTIN® (gabapentin) capsules, for oral use When prescribing NEURONTIN, carefully evaluate patients for a history of drug abuse and observe them for signs and symptoms of gabapentin misuse or abuse (e.g., self-dose escalation and drug-seeking behavior). Note: If you need help accessing information in different file formats, see . Language Assistance Available: | | | | | | | | | | | | | | | Gabapentin (Neurontin) FDA-approved indications and off-label uses; gabapentin withdrawal and abuse potential; mechanism of action; how long it takes for gabapentin to start working. FDA approval history for Gralise (gabapentin) used to treat Postherpetic Neuralgia. Supplied by Depomed, Inc. Gabapentin is an anti-epileptic drug, also called an anticonvulsant. It is used to treat some types of seizures and nerve pain caused by shingles. Gabapentin and pregabalin are FDA-approved for a variety of uses include fibromyalgia and restless legs syndrome. Gabapentin was first approved in 1993 and pregabalin was Gralise (gabapentin) Tablets 300 mg and 600 mg Company: Abbott Products, Inc. Application No.: 022544 Approval Date: 01/28/2011 Persons with disabilities having problems accessing the PDF files below may call (301) 796-3634 for assistance. Approval Letter (s) (PDF) Summary Review (PDF) Officer/Employee List (PDF) Cross Discipline Team Leader Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. However, it was later discovered that gaba
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |