Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 | |
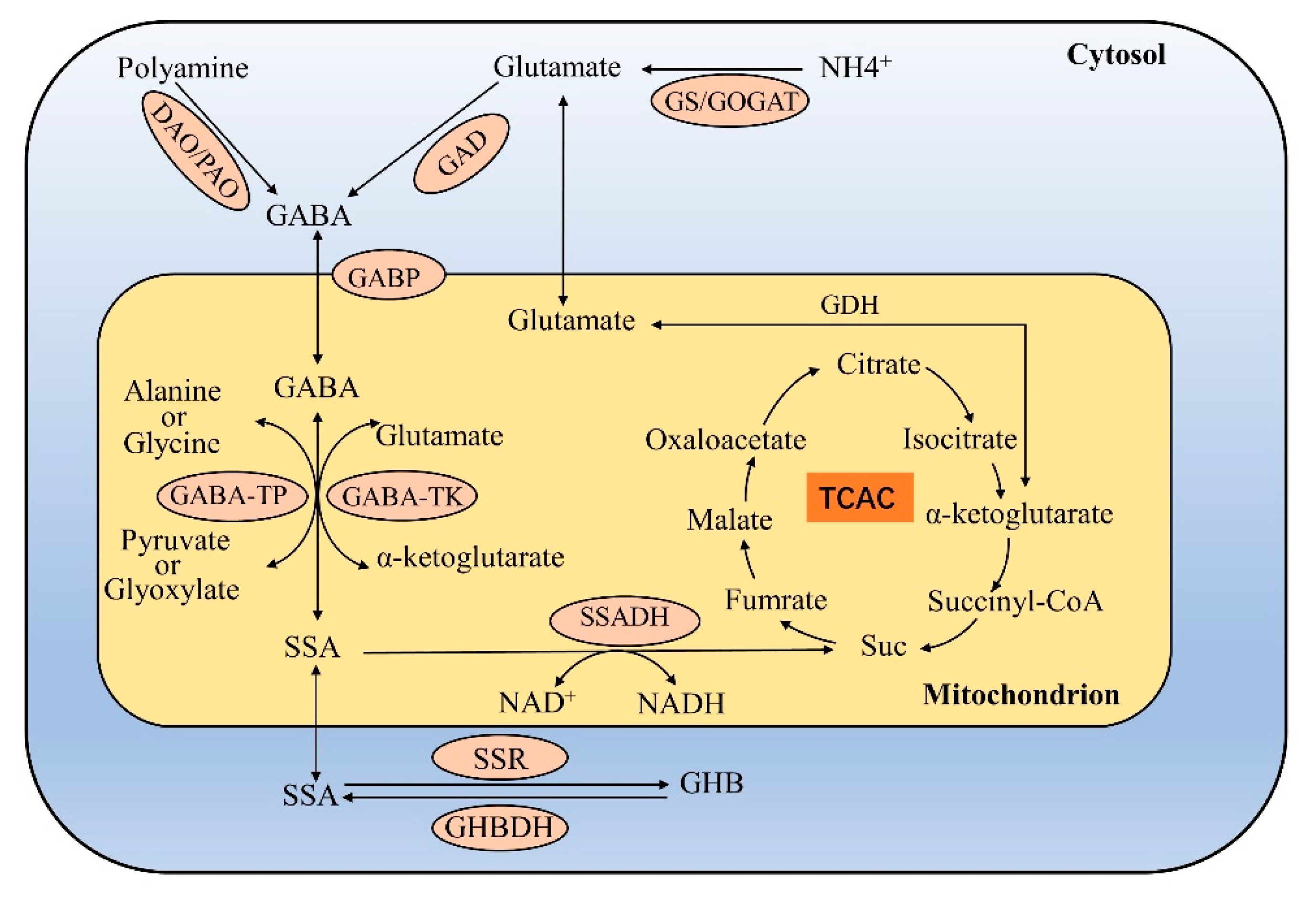 |
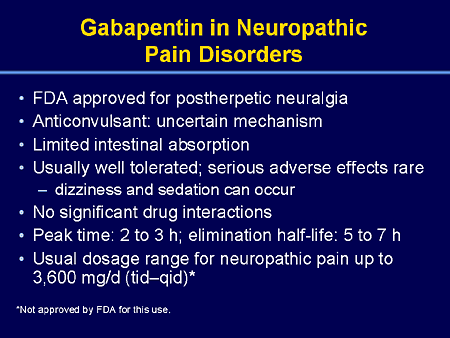
Abstract Gabapentin is approved for the treatment of postherpetic neuralgia (PHN) and epilepsy. The pharmacokinetic (PK) properties of gabapentin, including absorption, distribution, metabolism, and excretion (ADME), were investigated during the development of Neurontin®, an immediate-release (IR) formulation of gabapentin that is orally administered three-times daily. Recently, a This paper describes the pharmacokinetic studies of 1-(aminomethyl)-cyclohexane acetic acid (gabapentin, Gö 3450, CI-945) conducted with the 14C-labelled substance following intravenous and intragastric administration to rats and dogs and oral administration to humans. Gabapentin is well absorbed in Gabapentin (“Neurontin”) has been shown in extensive preclinical and clinical studies to be an effective anticonvulsant drug, which appears to have novel mechanisms of action. Although designed as a GABA analogue it is clearly not GABAmimetic, although The metabolism of gabapentin has not been studied in cats, but pharmacokinetics demonstrates faster elimination than in humans, with similar implications for dose intervals as in dogs. 3 Additionally, gabapentin may modulate the activity of various enzymes involved in the metabolism of neurotransmitters, further contributing to its multifaceted action. Pharmacokinetically, gabapentin has a unique absorption profile. Summary: This paper describes the pharmacokinetic studies of 1-(aminomethyl)-cyclohexane acetic acid (gabapentin, Go 3450, CI-945) conducted with the '4C-labelled substance fal-lowing intravenous and intragastric administration to rats and dogs and oral administration to humans. Gabapentin is well absorbed in rats, dogs and in humans, with maximum blood levels, reached within 1-3 h after Gabapentin is not protein-bound. A high volume of distribution indicates greater concentration in tissue than in plasma. It is not metabolized and does not induce hepatic enzymes or inhibit metabolism of other antiepileptic drugs. Structure of GABA: gabapentin and pregabalin. 10 Pharmacokinetics The actions of gabapentinoids are mainly at an intracellular site and require active uptake. They undergo facilitated transport across cell membranes through system l -amino acid transporters (LAT) as both drugs are structurally similar to the amino acid leucine. The effects of chronic gabapentin are blocked by an inhibitor of Gabapentin is a new antiepileptic drug (AED) with an attractive pharmacokinetic profile. It is absorbed by an active and saturable transport system, and has a high volume of distribution. Gabapentin is not bound to plasma proteins, does not induce hepatic enzymes and is not metabolized. At steady st Gabapentin enacarbil is licensed for restless leg syndrome in the United States.17 GBP-GR is adminis-tered once daily and gabapentin enacarbil is adminis-tered in two divided doses.18 GBP-GR exhibits saturable absorption similar to immediate-release gabapentin but this is enhanced by high-fat content in meals.18 Pharmacokinetic comparisons show The new antiepileptic medications are prescribed for the treatment of patients with seizure disorders since 17 years ago. Gabapentin (GBP) was approved on January 1994 as adjunctive treatment in patients 12 years or older with partial seizures, with Gabapentin's elimination involves renal clearance, with minimal metabolism by the liver. Its half-life is typically 5-7 hours, but can vary based on renal function. Clinical factors like dosage, individual metabolism, and renal impairment can influence its clearance. Analytical techniques, such as blood tests, are used to monitor drug levels and assess metabolism. Gabapentin is an anticonvulsant medication used in the management of peripheral neuropathic pains, postherpetic neuralgia, and partial-onset seizures. In addition, gabapentin has a relative lack of drug–drug interactions, which makes it a desirable adjunctive agent in the management of epilepsy in patients taking multiple medications. A low degree of protein binding and freedom from hepatic metabolism also make it an attractive agent in patients with hepatic impairment. After oral administration and absorption, gabapentin circulates essentially unbound to serum proteins. In addition, gabapentin does not undergo hepatic metabolism, unlike most other antiepileptic drugs, and is eliminated almost entirely by renal excretion with a clearance that approximates the glomerular filtration rate. Gabapentin is an anticonvulsant drug effective in humans to control neuropathic pain. In veterinary medicine, is extra-label used in combination with other treatments to control seizures when other drugs are not effective, when drugs are toxic, or Pharmacodynamics Mechanisms of action Gabapentin and pregabalin do not bind to GABA receptors despite their structural similarity but have a high affinity for the α2δ-1 subunit of voltage-gated In vitro studies were conducted to investigate the potential of gabapentin to inhibit the major cytochrome P450 enzymes (CYP1A2, CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4) that mediate drug and xenobiotic metabolism using isoform selective marker substrates and human liver microsomal preparations. Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. However, it was later discovered that gabapentin has the potential of an anticonvulsive medication and can be used as an adjunct to more
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 | |
 |