Gallery
Photos from events, contest for the best costume, videos from master classes.
 | 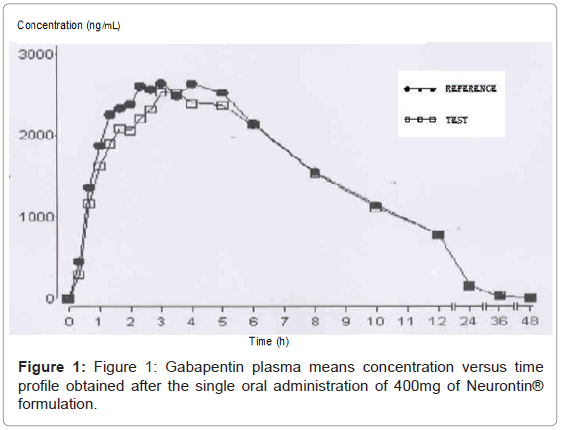 |
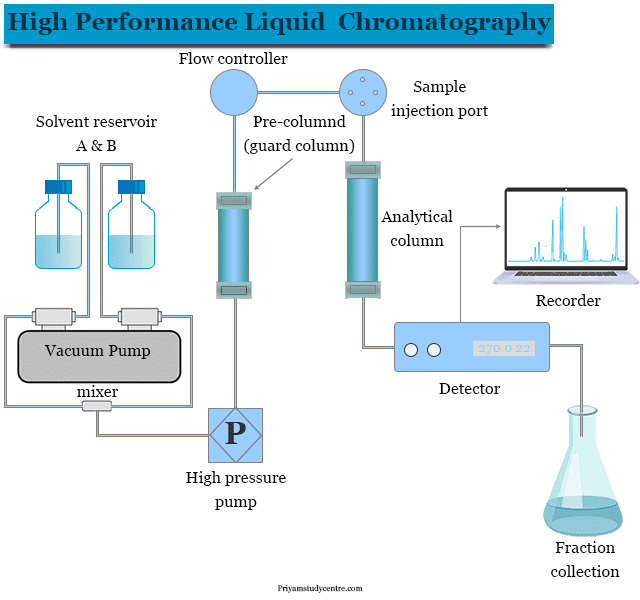 |  |
 |  |
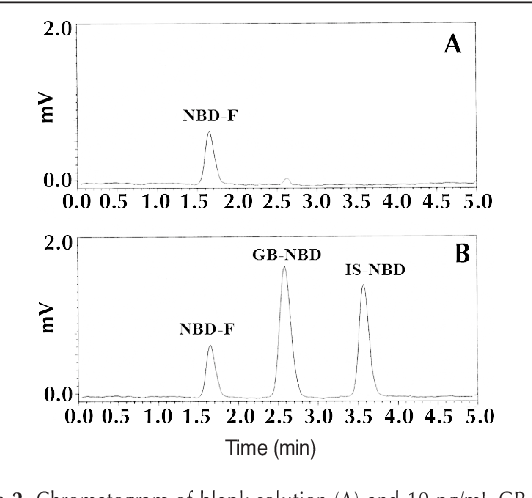 |  |
 | 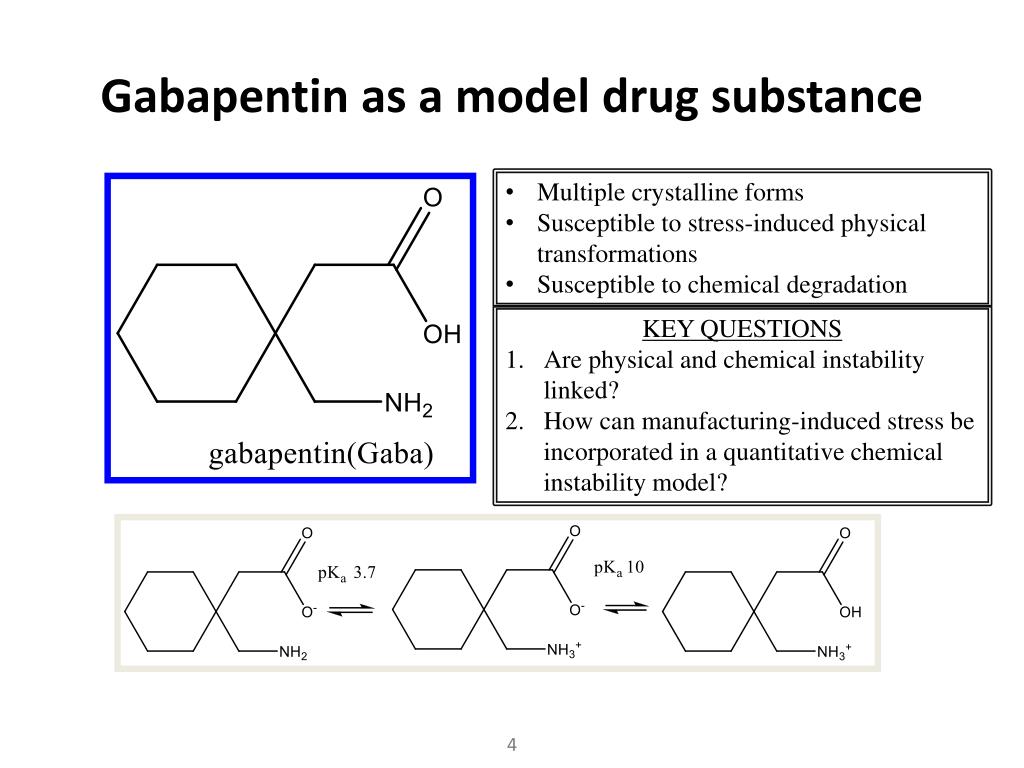 |
 |  |
Gauthier D, Gupta R (2002) Determination of gabapentin in plasma by liquid chromatography with fluorescence detection after solid-phase extraction with a C18 column. A simple and rapid liquid chromatography-tandem mass spectrometric method is developed and validated for the determination of gabapentin in human plasma. Metformin is used as an internal standard. A rapid, sensitive and specific analytical method was developed and validated to quantify gabapentin in human plasma using acetaminophen as an internal standard. The method employs a single plasma protein precipitation. The analytes are chromatographed on a C4 reversed-phase chromatographic column a ared quickly within 10 min. To furthur improve chromatographic selectivity, the derivatives are concentrated on a thin C-18 solid-phase membrane and interferences are washed away. The retained purified derivatives are eluted from the membrane with a small volume of solvent and the eluate is directly injected onto an Ultrasphere C-18 high-performance liquid chromatography column with In this article an analytical method developed to detect N-Nitrosodibutylamine (NDBA) in Gabapentin Capsules (100 mg, 300 mg, and 400 mg) using Liquid Chromatography-High Resolution Mass CONFIRMATION OF PREGABALIN AND GABAPENTIN BY LIQUID CHROMATOGRAPHY – TANDEM MASS SPECTROMETRY 48.1 METHOD This test method may be used to confirm the presence of gabapentin (GABA) and pregabalin (PREG) in biological specimens. An accurate, highly sensitive, and precise method for quantitative analysis of tramadol (TMD) and gabapentin (GBP) by high performance liquid chromatography and tandem mass spectrometry in human plasma was proposed and validated successfully using An analytical method for the determination of gabapentin in serum obtained from venous blood samples has been developed using high-performance liquid chromatography (HPLC)–tandem mass spectrometry. In addition, a comparative study between capillary plasma samples and venous serum samples was carried out. This demonstrates the potential for the use of the described analytical system using Several analytical methods were used to quantify gabapentin such as dried plasma spots [10], gas chromatography mass spectrometry (GC-MS) [11], capillary electrophoresis [12], fluorometry [13] and high-performance liquid chromatography (HPLC) [14]; US Pharmacopoeia has a monograph to analyze valproic acid using HPLC method [15]. The Charged Aerosol Detector (CAD) is a detector used in conjunction with high-performance liquid chromatography (HPLC) and ultra high-performance liquid chromatography (UHPLC) to measure the amount of chemicals in a sample by creating charged aerosol particles which are detected using an electrometer. A rapid, sensitive and accurate high performance liquid chromatography with UV detection method was developed and validated for the quantification of gabapentin in bulk, pharmaceutical formulation and human urine samples. Most of the published 35.1 POLICY This test method may be used to confirm the presence of gabapentin (GABA) in biological samples. Quantitative results obtained through the use of this method will only be reported within the validated dynamic range. Reporting of results following the application of this method will be contingent upon a thorough review and acceptance of quality control data and the qualification of Abstract Rationale: Gabapentin has shown initial promise as an opioid-sparing medication in pain patients as well as a treatment for opioid withdrawal and liquid chriomatography/tandem mass spectrometry (LC/MS/MS) is often used for clinical monitoring. Despite reports of validated tandem mass spectrometric methods for the determination of gabapentin and buprenorphine, mechanisms for the A simple HPLC method was developed and validated for quantitation of gabapentin in pure form. The HPLC separation was achieved on a C18 5 μm Waters column (150 mm × 4.6 mm) using a mobile phase of methanol - potassium dihydrogen orthophosphate Abstract A sensitive high-performance liquid chromatography (HPLC) method using UV detection for the determination of gabapentin in human plasma has been developed. In this method, gabapentin was extracted from human plasma with a reversed-phase solid-phase extraction (SPE) cartridge followed by derivatization with phenylisothiocyanate. A simple, sensitive and rapid liquid chromatography/tandem mass spectrometry (LC–MS/MS) method was developed and validated for the quantification of gabapentin, a new antiepileptic drug, in human plasma using its structural analogue, 1,1-cyclohexane diacetic acid monoamide (CAM) as internal standard. The method involved a simple protein precipitation by means of acetonitrile followed by a Gabapentin and pregabalin are anticonvulsant drugs that are also utilized for pain management. A mass spectrometry method was developed and validated to quantify gabapentin and pregabalin in urine to support testing for adherence. 35.1 METHOD This test method may be used to confirm the presence of gabapentin (GABA) in biological specimens. GABA and internal standard (GABA-d10) are isolated from biological matrices by protein precipitation and solid-phase extraction (SPE). The extracts are injected into a high performance liquid chromatograph (HPLC) coupled to a mass spectrometer (MS) detector equipped with an Ultra-High Performance Liquid Chromatography (UHPLC) for Quantification of Gabapentin in Granules and Capsules Ultra-High Performance Liquid Chromatography (UHPLC) for Quantification of Gabapentin A rapid, sensitive and selective method for the determination of gabapentin in human plasma was developed using hydrophilic interaction liquid chromatography/tandem mass spectrometry (HILIC/MS/MS). The devised method involved protein precipitation with acetonitrile followed by separation on an Atlan
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |