Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
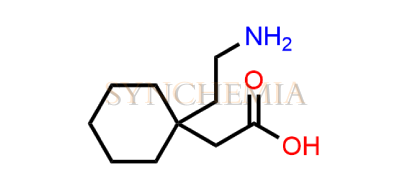
. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012 Update on gabapentin therapy of neuropathic pain. [Consult Pharm. 2003] Guay DR. Consult Pharm. 2003 Feb; 18 (2):158-70, 173-8. Gabapentin is a first line drug for the treatment of neuropathic pain in spinal cord injury. [Spine (Phila Pa 1976). 2004] Abstract Introduction: Gabapentin belongs to a class of drugs known as gabapentinoids. Due to its potent inhibitary effect in neuropathic pain, gabapentin became an attractive non-opioid adjunt treatment option. Gabapentin shows a mild-side effect profile with common adverse effects being somnolence, dizziness, headaches and peripheral edema. LiverTox® provides regularly updated, unbiased and easily accessed information on the diagnosis, cause, frequency, clinical patterns and management of liver injury attributable to prescription and nonprescription medications and selected herbal and dietary supplements. The LiverTox site is meant as a resource for both physicians and patients as well as for clinical academicians and Therefore, an understanding of liver enzyme levels, often measured via tools such as liver function tests (LFTs), is crucial for patients on gabapentin. The question of whether is gabapentin bad for your liver requires careful evaluation, particularly for individuals with pre-existing hepatic conditions. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Show details Drug Records < Prev Next > Agents Included in LiverTox by Drug Class Last Update: January 30, 2025. As in other cases of DILI, gabapentin-induced hepatotoxicity occurs within the first seven days post exposure to the drug. The discontinuation of gabapentin leads to an improvement in liver test abnormalities. The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Anticonvulsant medications include many agents that have been incriminated in causing idiosyncratic drug induced liver disease. Indeed, several commonly used anticonvulsants (phenytoin, valproate, carbamazepine) are consistently ranked in the top causes of clinically apparent drug induced liver injury and are frequently listed in causes of drug induced acute liver failure. Because of the Abstract Background & aims: Antiepileptic drugs (AEDs) are a common cause of drug-induced liver injury (DILI). Over the last few decades, several newer AEDs were approved for marketing in the United States, and they are increasingly prescribed for indications other than seizures. Contemporaneous data related to trends and characteristics of AED-related liver injury are sparse. Introduction: Gabapentin is an anti-convulsant that is also used off-label to treat neuropathic pain. It is not metabolized by the liver, and there have been few reports of hepatotoxity associated with it. We present a rare case of gabapentin-induced hepatotoxicity occurring in a young male. Case Description/Methods: A 41-year-old male with an extensive past medical history including type 1 Gabapentin enacarbil is a long acting form of gabapentin that is used for restless leg syndrome and for painful postherpetic neuropathy. Gabapentin enacarbil and gabapentin are associated with a low rate of transient serum enzyme elevations during treatment and with rare instances of clinically apparent liver injury. LiverTox<sup>®</sup> provides regularly updated, unbiased and easily accessed information on the diagnosis, cause, frequency, clinical patterns and management of liver injury attributable to prescription and nonprescription medications and selected herbal and dietary supplements. The LiverTox site i Gabapentin doesn’t hurt the liver or kidneys in most cases. However, taking a safe gabapentin dose is important to prevent potential side effects. Of 5 LiverTox DILI likelihood Category A AEDs (phenytoin, carbamazepine, valproate, lamotrigine, and fosphenytoin), the number of annual prescriptions for phenytoin, carbamazepine, and valproate have drastically reduced between 2004 and 2018 (Figure 1). Gabapentin, which has been FDA-approved for the treatment of postherpetic neuralgia in adults, is commonly used for this disorder. Gabapentin, a common over-the-counter pain reliever and fever reducer, has been linked to rare individual case reports of liver injury. The causal relationship between gabapentin and liver damage is unclear, with the latency to onset being 1 to 8 weeks. Tylenol, a common over-the-counter pain reliever, is not toxic to the liver when taken in moderation but can cause liver damage when used in Gabapentin enacarbil is a long acting form of gabapentin that is used for restless leg syndrome and for painful postherpetic neuropathy. Gabapentin enacarbil and gabapentin are associated with a low rate of transient serum enzyme elevations during treatment and with rare instances of clinically appa Gabapentin is a unique anticonvulsant that is used as adjunctive therapy in management of epilepsy and for neuropathic pain syndromes. Therapy with gabapentin is not associated with serum aminotransferase elevations, but several cases of clinically apparent liver injury from gabapentin have been rep LiverTox is a web-based resource for information on drug-induced liver injury from prescription and OTC drugs, and complementary and alternative medicines. Request PDF | Gabapentin-associated hepatotoxicity | The American Journal of Gastroenterology is published by Nature Publishing Group (NPG) on behalf of the American College of Gastroenterology Pregabalin is an inhibitor of neuronal activity used for therapy of painful neuropathy and as an anticonvulsant. Therapy with pregabalin is not associated with serum aminotransferase elevations, and clinically apparent liver injury from pregabalin has been reported but appears to be quite rare.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |