Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 | |
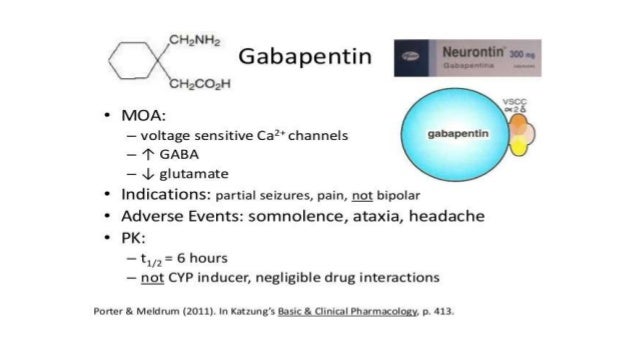 | 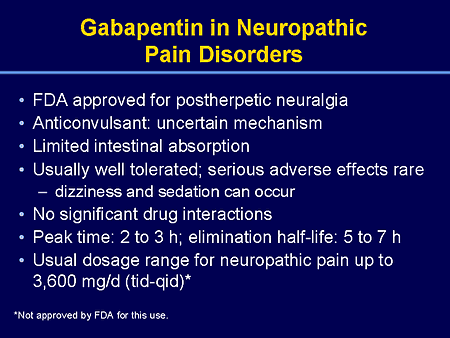 |
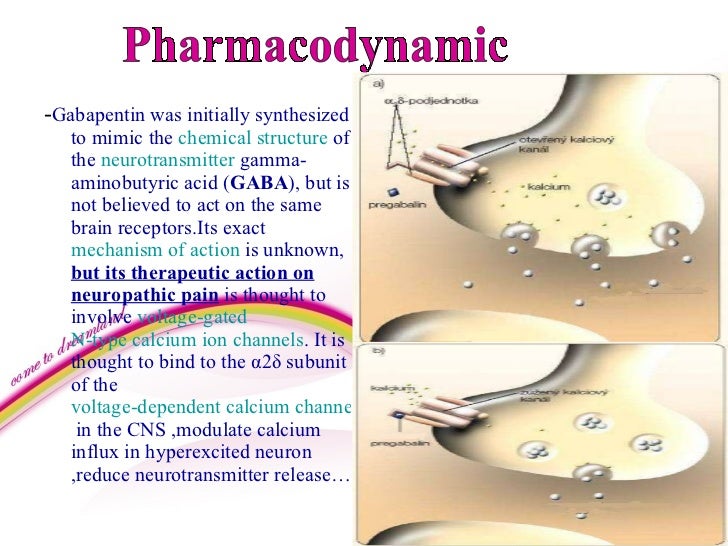
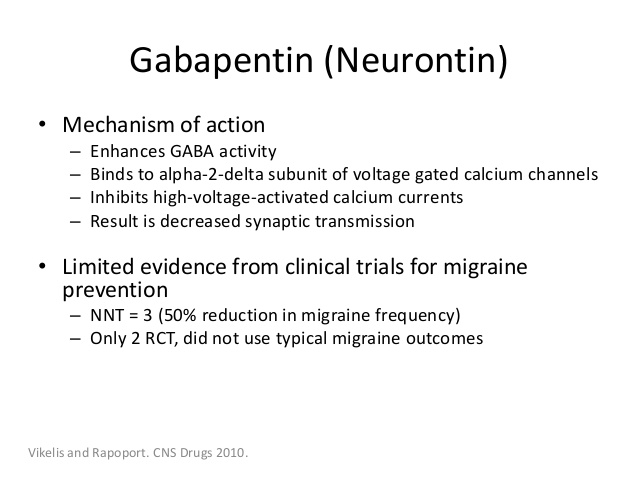
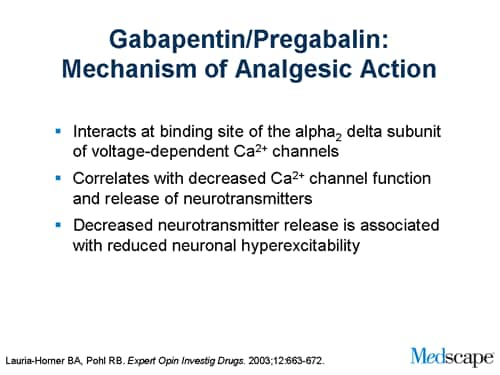
Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. However, it was later discovered that gabapentin has the potential of an anticonvulsive medication and can be used as an adjunct to more potent anticonvulsants. The medication also proves beneficial in managing certain types of neural pain and psychiatric disorders. Gabapentin also fails to inhibit the internalization rate of α 2 δ ‐2 but does disrupt rab11‐dependent recycling from endosomal compartments consequently reducing calcium currents through this mechanism (Tran‐Van‐Minh and Dolphin 2010). Gabapentin has become popular as a first-line treatment for neuropathic pain because of its efficacy as an antineuropathic agent and relatively benign side-effect profile. However, its mechanism of action is far from clear. This review discusses the available evidence for the postulated mechanisms of action of gabapentin. General Description Gabapentin is an analgesic medication commonly used for neuropathic pain. It exerts its effects through various mechanisms of action, including the inhibition of voltage-gated calcium channels (VGCCs) and disruption of α2δ-1-NMDAR complexes. By reducing the release of excitatory neurotransmitters and blocking synaptogenesis, gabapentin helps alleviate neuropathic pain. It Gabapentin is an anti-epileptic agent but now it is also recommended as first line agent in neuropathic pain, particularly in diabetic neuropathy and post herpetic neuralgia. α2δ-1, an auxillary subunit of voltage gated calcium channels, has been documented as its main target and its specific bindin Gabapentin is commonly used to treat neuropathic pain (pain due to nerve damage). This review updates a review published in 2014, and previous reviews published in 2011, 2005 and 2000. To assess the analgesic efficacy and adverse effects of The gabapentinoids, pregabalin and gabapentin, have been the cornerstone of pharmacological management of neuropathic pain.1 Despite the widespread use in neuropathic pain, the precise mechanism of action is uncertain. The effect of gaba-pentinoids in pain are assumed to be because of direct inhibi-tion of voltage gated Ca2þ channels by binding to its a2d-1 subunit resulting in reduction of Gabapentin, sold under the brand name Neurontin among others, is an anticonvulsant medication primarily used to treat neuropathic pain and also for partial seizures [10][7] of epilepsy. Gabapentin or an α2δ-1 C terminus-interfering peptide normalizes NMDAR synaptic targeting and activity increased by nerve injury. Thus, α2δ-1 is an NMDAR-interacting protein that increases NMDAR synaptic delivery in neuropathic pain. Gabapentinoids reduce neuropathic pain by inhibiting forward trafficking of α2δ-1-NMDAR complexes. Includes Gabapentin indications, dosage/administration, pharmacology, mechanism/onset/duration of action, half-life, dosage forms, interactions, warnings, adverse Gabapentin prevents pain responses in several animal models of hyperalgesia and prevents neuronal death in vitro and in vivo with models of the neurodegenerative disease amyotrophic lateral sclerosis (ALS). Gabapentin is also active in models that detect anxiolytic activity. Gabapentin is an anticonvulsant medication used in the management of peripheral neuropathic pains, postherpetic neuralgia, and partial-onset seizures. Medical Indications In animal models of analgesia, gabapentin prevents allodynia and hyperalgesia. Gabapentin is indicated for: Neuropathic pain caused by postherpetic neuralgia Adjunctive therapy in the treatment of partial seizures with or without secondary generalization Neuropathic pain caused by diabetic peripheral neuropathy and spinal cord injury Restless leg syndrome (gabapentin Gabapentin (GBP) was originally developed as a potential agonist for Gamma-Amino-Butyric-Acid (GABA) receptors, aiming to inhibit the activation of pain-signaling neurons. Contrary to initial expectations, it does not bind to GABA receptors. Instead, it exhibits several distinct pharmacological activities, including: (1) binding to the alpha-2-delta protein subunit of voltage-gated calcium Gabapentin is an anti-epileptic agent but now it is also recommended as first line agent in neuropathic pain, particularly in diabetic neuropathy and post herpetic neuralgia. α2δ-1, an auxillary subunit of voltage gated calcium channels, has been documented as its main target and its specific binding to this subunit is described to produce different actions responsible for pain attenuation The gabapentinoids, pregabalin and gabapentin, have been the cornerstone of pharmacological management of neuropathic pain. 1 Despite the widespread use in neuropathic pain, the precise mechanism of action is uncertain. The effect of gabapentinoids in pain are assumed to be because of direct inhibition of voltage gated Ca 2+ channels by binding to its α2δ-1 subunit resulting in reduction of Keywords: Gabapentin, pregabalin, pain management, adverse effects, pharmacology Introduction The gabapentinoid drugs gabapentin and pregabalin are antiepileptic drugs that are considered as first-line treatments for the management of neuropathic pain. 1 Pregabalin is also approved for generalised anxiety disorders in the United Kingdom. Gabapentin [1- (aminomethyl)cyclohexane acetic acid] is␣a␣novel anti-epileptic agent, originally developed as a gamma-aminobutyric acid (GABA)-mimetic compound to treat spasticity, and has been shown to have potent anticonvulsive effects [1, 2]. Initially approved only for use in partial seizures, it soon showed promise in the treatment of chronic pain syndromes, especially neuropathic Gabapentin has been clearly demonstrated to be effective for the treatment of neuropathic pain in diabetic neuropathy and postherpetic neuralgia. This evidence, combined with its favourable side-effect profile in various patient groups (including the elderly) and lack of drug interactions, makes it an attractive agent. Gabapentinoids, including gabapentin and pregabalin, are extensively used for treatment of neuropathic pain, restless legs syndrome, and focal seizures. Their efficacy in these disorders is primarily attributed to their effects in inhibiting the functions of the α2δ subunit of presynaptic VGCCs, thereby reducing neurotransmitter release.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 | |
 |  |