Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 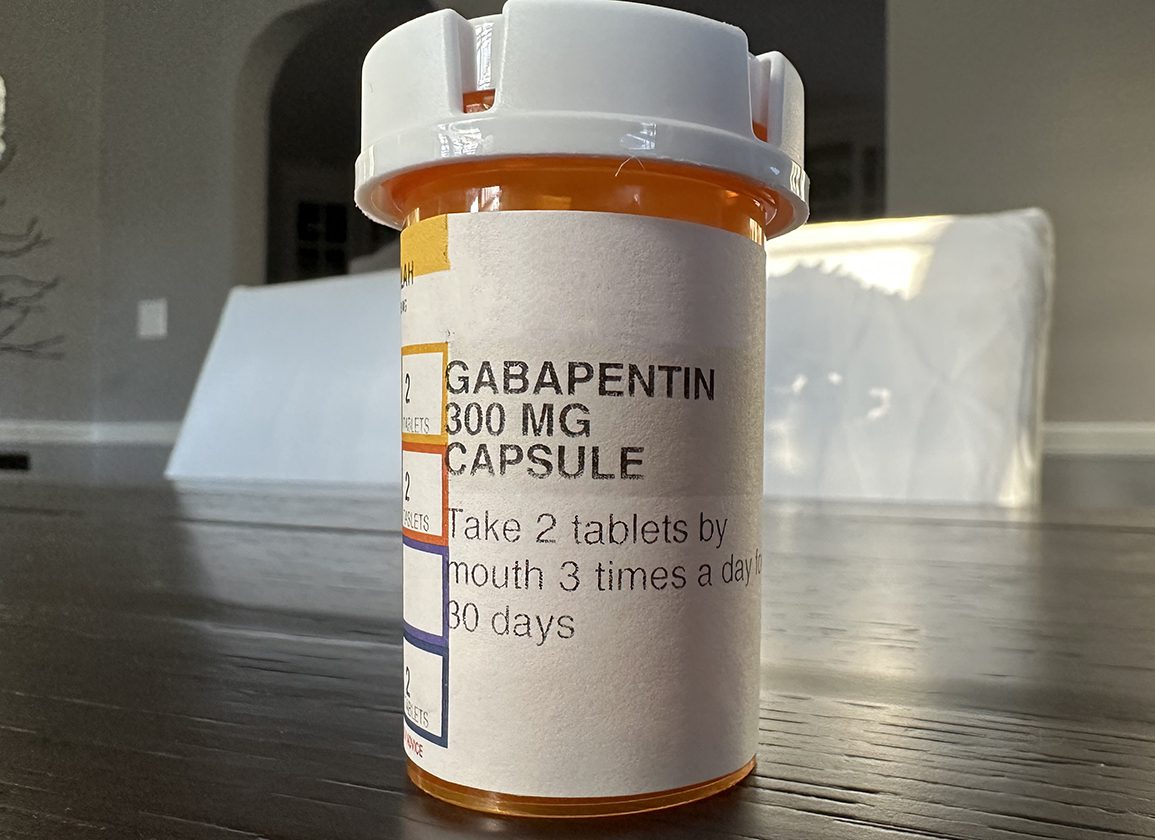 |
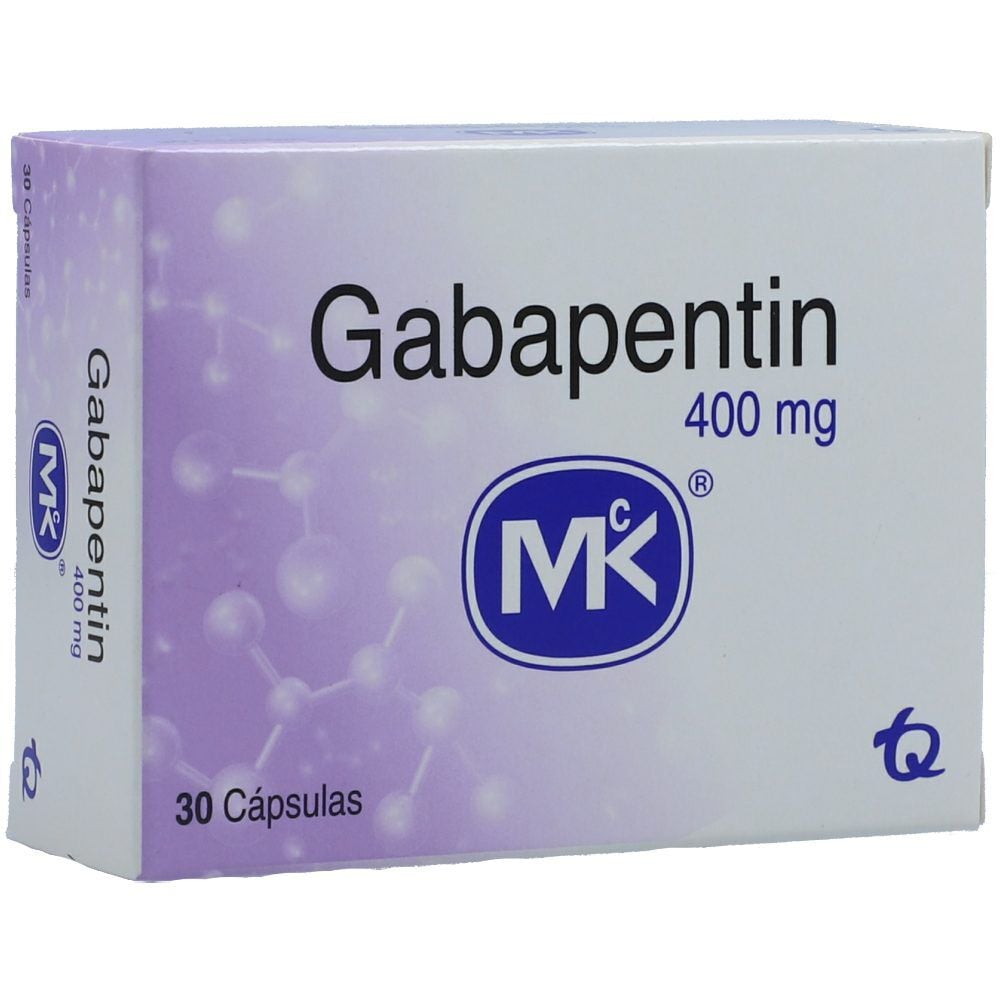
Information on drug and health products authorized by Health Canada. A double-blind, randomized, two-way crossover, single-dose (1 x 400 mg) bioequivalence study of GABAPENTIN 400 mg capsules (Teva Canada Limited) and NEURONTIN® 400 mg capsules (Upjohn EESV) was conducted in 38 healthy adult male subjects under fasting conditions. Drug and Health Product Portal Information on drugs and health products authorized by Health Canada. Gabapentin treatment prevented tonic extensor seizures in mice from a variety of convulsant agents, including bicuculline, picrotoxin, strychnine and thiosemicarbazide. PRODUCT MONOGRAPH PrNEURONTIN® Gabapentin Capsules 100 mg, 300 mg, and 400 mg Tablets 600 mg and 800 mg Upjohn Canada ULC 17300 Trans-Canada Highway Kirkland, Quebec H9J 2M5 Submission Control No.: 237475 ®Warner-Lambert Company LLC Upjohn Canada ULC, Licensee Upjohn Canada ULC, 2020 Antiepileptic Agent Date of Revision: May 5, 2020 RIVA-GABAPENTIN (gabapentine) est indiqué comme traitement adjuvant chez les patients dont l’état épileptique n’est pas stabilisé de façon satisfaisante par la thérapeutique classique. This leaflet is part III of a three-part “Product Monograph" published when Neurontin was approved for sale in Canada and is designed specifically for Consumers. IMPORTANT: PLEASE READ This leaflet is part III of a three-part “Product Monograph” published when GABAPENTIN was approved for sale in Canada and is designed specifically for Consumers. This leaflet is a summary and will not tell you everything about GABAPENTIN. Contact your doctor or pharmacist if you have any questions about the drug. The product monograph is developed by a drug sponsor according to guidelines published by Health Canada that provide direction on the content and format. The veterinary labelling is developed by the drug sponsor according to the Food and Drug Regulations. While Health Canada reviews the product monograph or the veterinary labelling as part of the drug review process, it remains the The product monograph is developed by a drug sponsor according to guidelines published by Health Canada that provide direction on the content and format. The veterinary labelling is developed by the drug sponsor according to the Food and Drug Regulations. While Health Canada reviews the product monograph or the veterinary labelling as part of the drug review process, it remains the Information on drugs and health products authorized by Health Canada. Click on a resource to visit a page with more information. You may be taken away from this page to a different Government of Canada website. PART I: HEALTH PROFESSIONAL INFORMATION 1 INDICATIONS Adults TEVA-GABAPENTIN (gabapentin) is indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. This leaflet is part III of a three-part “Product Monograph" published when TEVA-GABAPENTIN was approved for sale in Canada and is designed specifically for Consumers. A randomized, two-treatment, two-sequence, single dose, crossover bioequivalence study of Gabapentin, 600mg (JAMP Pharma Corporation) with Neurontin® (gabapentin) tablets, 600mg (Pfizer Canada Inc., Canada) was conducted in 32 healthy human adult male and female subjects, under fasting conditions. NEURONTIN (gabapentin) belongs to the family of medicines called antiepileptic drugs and is used for treating epilepsy (seizures). NEURONTIN has been prescribed for you by your doctor to reduce your number of seizures. This leaflet is part III of a three-part “Product Monograph" published when MYLAN-GABAPENTIN was approved for sale in Canada and is designed specifically for Consumers. This leaflet is part III of a three-part "Product Monograph" published when GABAPENTIN was approved for sale in Canada and is designed specifically for Consumers. Gabapentin Capsules Capsules, 100 mg, 300 mg and 400 mg, oral Search the Drug Product Database (DPD) to find drugs authorized for sale by Health Canada. The DPD is updated nightly and includes: Generic drug manufacturers must update their PM to ensure it aligns with the Canadian Reference Product. NEURONTIN® (gabapentin) Product Monograph management of side effects, contact your health professional. The Canada Vigilance Program does not provide medical advice.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |