Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
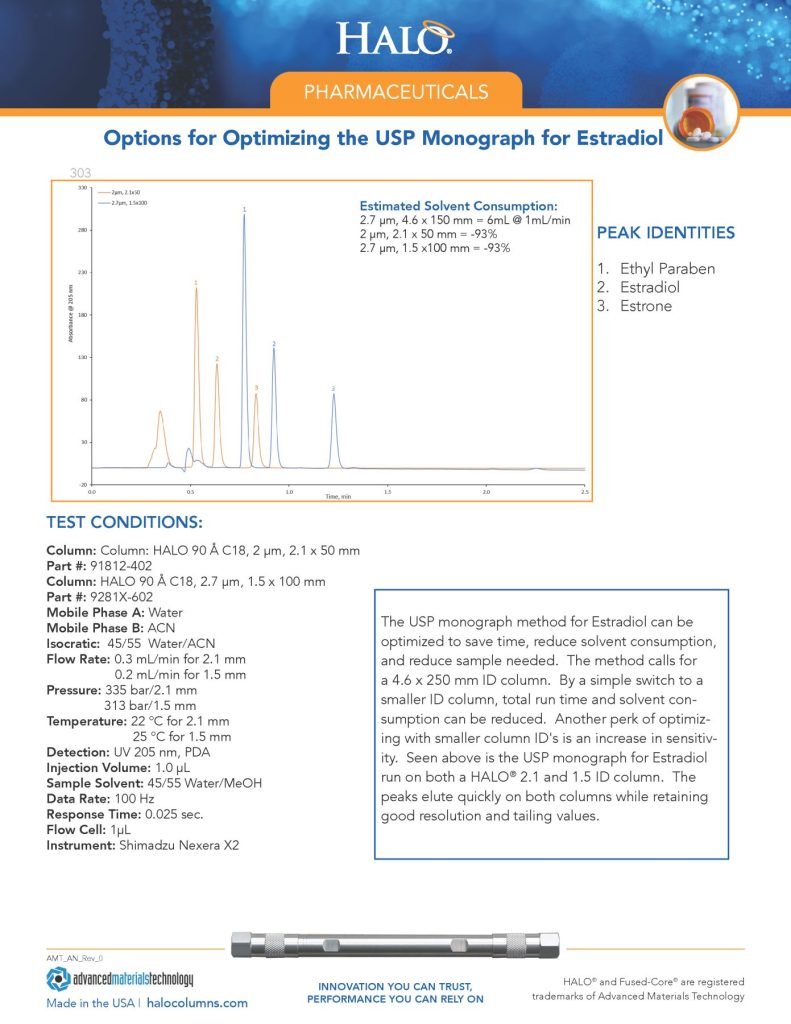
Gabapentin tablets, USP are indicated as adjunctive therapy in the treatment of partial seizures with and without secondary generalization in patients over 12 years of age with epilepsy. MYLAN-GABAPENTIN (gabapentin) is indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. Systematic studies in geriatric patients have not been conducted. (See WARNINGS AND PRECAUTIONS, Special Populations). USP 35 Procedure—Separately inject equal volumes (about 20 mL) of the Standard solution and the Test solution into the chromato- graph, record the chromatograms, and measure the responses for the major peaks. [ NOTE—Disregard all the peaks having rela- tive retention times of 0.35 or less relative to gabapentin re- lated compound D, as these are quantified in the test for Limit of Early Diluent, Buffer solution, Mobile phase, and Chromatographic system— Proceed as directed in the Assay. Impurities solution— Dissolve suitable quantities of USP Gabapentin Related Compound A RS and USP Gabapentin Related Compound B RS in methanol to obtain a solution containing about 1.4 mg per mL and 0.84 mg per mL, respectively. Test solution—Use the Assay preparation. Standard solution—Dissolve a suitable quantity of USP Gabapentin Related Compound E RS in Diluent to obtain a solu-tion having a known concentration of 8.4 mg per mL. Chromatographic system (see Chromatography á621ñ)—Pre-pare as directed in the Assay. Gabapentin is used in combination with other anticonvulsants for management of partial seizures with or without secondary generalization in adults and children ≥3 years of age. United States Pharmacopeia (). USP Monographs, Gabapentin Tablets. . Rockville, MD: United States Pharmacopeia. United States Pharmacopeia (2021). USP Monographs, Gabapentin Compounded Oral Suspension. USP-NF. Rockville, MD: United States Pharmacopeia. Calculate the amount of gabapentin (C 9 H 17 NO 2) dissolved by the formula: in which rU and rS are the peak responses for the Working standard solution and the Test solution, respectively; CS is the concentration, in mg per mL, of the Working standard solution; 900 is the volume, in mL, of Medium; 100 is the conversion factor to percentage Gabapentin USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monographs such as: The Gabapentin Tablets Revision Bulletin supersedes the monograph in USP 32–NF 27 until it is printed in the USP 33–NF 28 First Supplement which will be released 1 February 2010 and becomes official 1 August 2010. View the USP Certificate or Product Information Sheet in the table above to view additional product details, including available label text and storage information. USP Monographs, Gabapentin. USP-NF. Rockville, MD: United States Pharmacopeia. Gabapentin Capsules contain NLT 90.0% and NMT 110.0% of the labeled amount of gabapentin (C 9 H 17 NO 2). United States Pharmacopeia (). USP Monographs, Gabapentin Capsules. USP-NF. Rockville, MD: United States Pharmacopeia. Gabapentin Tablets » Gabapentin Tablets contain not less than 90.0 percent and not more than 110.0 percent of the labeled amount of gabapentin (C9H17NO2). l-closed containers. Store at control USP Gabapentin RS. USP Gabapentin R Product Monograph – Neurontin® (gabapentin) capsules 100 mg, 300 mg, and 400 mg and tablets 600 mg and 800 mg. Pfizer Canada Inc. Date of revision: February 22, 2018, Control No.:211678. This leaflet is part III of a three-part “Product Monograph" published when Gabapentin Capsules USP and Gabapentin Tablets USP was approved for sale in Canada and is designed specifically for Consumers. Neurontin (gabapentin) is indicated as adjunctive therapy in the treatment of partial seizures with and without secondary generalization in patients over 12 years of age with epilepsy. abapentin impairs their ability to drive. Driving performance studies conducted with a prodrug of gabapentin (gabapentin enacarbil tablet, extended-release) indicate that gabapentin may cause significant driving impairment. Prescribers and patients should be aware that patients’ ability to assess their own driving competence, as well as their United States Pharmacopeia (2025). USP Monographs, Gabapentin Tablets. USP-NF. Rockville, MD: United States Pharmacopeia.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |