Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
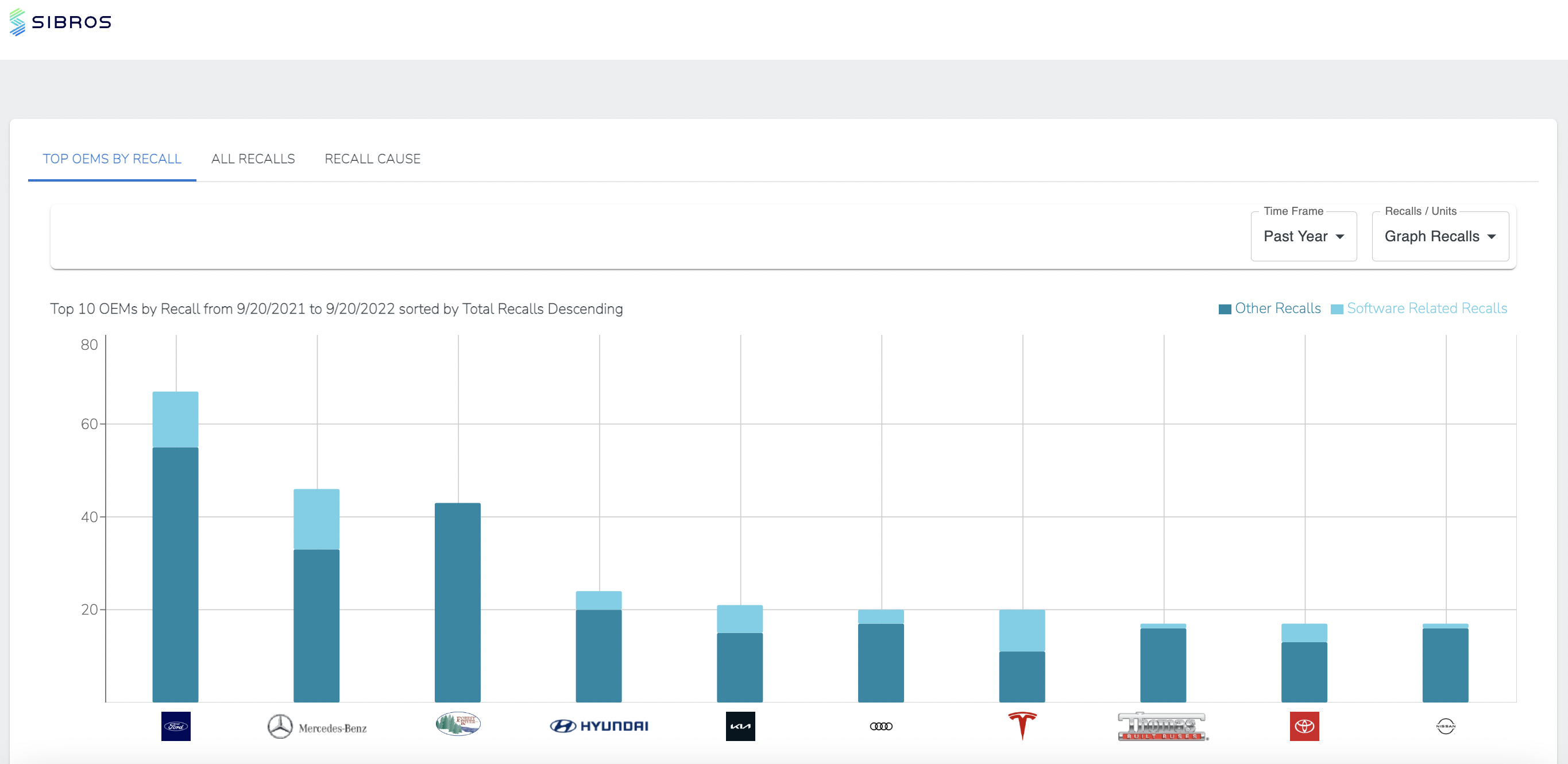
Glenmark Pharmaceuticals recalls nearly 40 lots of generic medications over manufacturing issues impacting treatments for common health conditions. The most recent Recall Enforcement Report that covers this product was initiated on July 31st, 2024 and classified as a Class II recall due to presence of foreign tablets; 3 fused tablets of metformin er 500 mg were found in bottle of gabapentin tablets This recall is currently terminated, and the associated recall number is recall number is D-0634-2024. It pertains to Gabapentin identified by The recall affects 13 728 bottles—12 876 bottles of 300 mg capsules and 852 bottles of 400 mg capsules—due to concerns of cross contamination during manufacturing. The impacted lots, with expiration dates ranging from March to August 2025, were distributed nationwide across the United States. Recalls, warnings, and alerts A warning that serious breathing problems may occur in patients using gabapentin (Neurontin, Gralise, Horizant) or pregabalin (Lyrica, Lyrica CR) who have respiratory risk factors. Drug Recall Enforcement Report Class II voluntary initiated by Granules Pharmaceuticals Inc., originally initiated on 07-31-2024 for the product Gabapentin Tablets, USP, 600 mg, 500-count bottles, Rx only, Manufactured by: Granules India Limited Hyderabad-500 081, India, Manufactured for: Granules Pharmaceuticals Inc., Chantilly, VA NDC 70010-227-05 The product was recalled due to presence of Disclaimer: All third-party product, company names and logos are trademarks™ or registered ® trademarks and remain the property of their respective holders. Usage, warnings, side effects, and community information for the prescription drug Gabapentin The U.S. Food and Drug Administration (FDA) has reported that Aurobindo Pharma USA is voluntarily recalling Gabapentin 300 mg capsules from lot GESB14011-A in 100-count bottles. The product lot affected was found to include some empty capsules; thus, if taken by a person with seizures, they would The FDA has reported out that, Sun Pharmaceutical Industries, Inc. initiated a recall for 13,728 bottles of gabapentin, including 12,876 bottles containing 300-milligram dosage and 852 bottles containing 400 milligrams per dose. Nearly 40 generic medications—most prescription, but some sold over-the-counter at Amazon and Walmart—have been recalled due to manufacturing quality concerns, the FDA said. See the full list GRANULES PHARMACEUTICALS INC. is recalling Gabapentin Tablets USP, 600mg. This recall is due to a potential mix-up of Metformin ER Tablets in a bottle of Gabapentin Tablets, USP 600mg. The ongoing investigation revealed that the issue is limited to the above lot and no other lots were impacted. No adverse events have been received to-date for the above mentioned product lot. Featured in this section are important public updates pertaining to our products, performance and business, along with print coverage in the media. Description: Gabapentin Tablets 600mg, 500-count bottles, Rx Only, Manufactured by: Glenmark Pharmaceuticals Limited, Pithampur, Madhya Pradesh 454775, India, Manufactured for: Glenmark Pharmaceuticals Inc. USA, Mahwah, NJ 07430. On July 31, 2024, Granules Pharmaceuticals Inc. recalled one lot of gabapentin 600mg tablets. The recall was initiated due to an error resulting in metformin extended-release tablets found in a bottle of gabapentin 600mg tablets. Reports of empty capsules prompt a nationwide voluntary recall of one lot of 300-mg capsules of the antiepileptic drug gabapentin. The U.S. Food and Drug Administration (FDA) has reported that Aurobindo Pharma USA is voluntarily recalling Gabapentin 300 mg capsules from lot GESB14011-A in 100-count bottles. The product lot affected was found to include some empty capsules; thus, if taken by a person with seizures, they would The FDA has reported out that, Sun Pharmaceutical Industries, Inc. initiated a recall for 13,728 bottles of gabapentin, including 12,876 bottles containing 300-milligram dosage and 852 bottles containing 400 milligrams per dose. The most recent Recall Enforcement Report that covers this product was initiated on July 31st, 2024 and classified as a Class II recall due to presence of foreign tablets; 3 fused tablets of metformin er 500 mg were found in bottle of gabapentin tablets This recall is currently completed, and the associated recall number is recall number is D-0634-2024. It pertains to Gabapentin identified by Drug Recall Enforcement Report Class III voluntary initiated by The Harvard Drug Group, originally initiated on 04-24-2023 for the product Gabapentin Tablets, USP 600 mg, packaged in Cartons of 100 tablets (10 tablets per blister pack x 10), Rx Only, Distributed by: Aurobindo Pharma USA, Inc. East Windsor, NJ 08520 Distributed by: Major Pharmaceuticals 17177 N Laurel Park Dr., Suite 233 On July 31, 2024, Granules Pharmaceuticals Inc. recalled one lot of gabapentin 600mg tablets. The recall was initiated due to an error resulting in metformin extended-release tablets found in a bottle of gabapentin 600mg tablets.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |