Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
12097-4/asset/b8e7d24e-0e2e-463d-967e-9e3ed3b43665/main.assets/gr1_lrg.jpg) |  |
 | |
 |  |
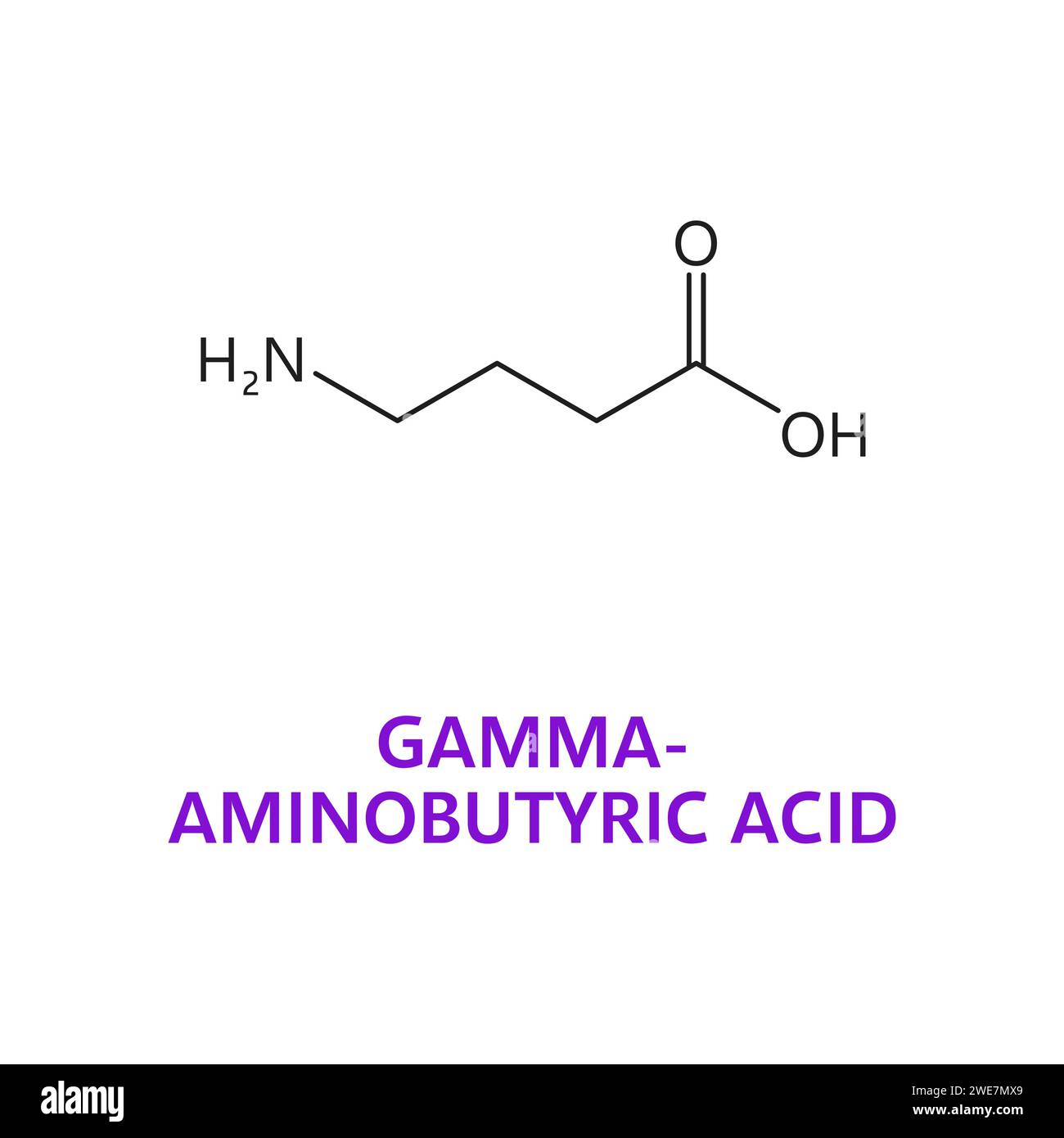 | 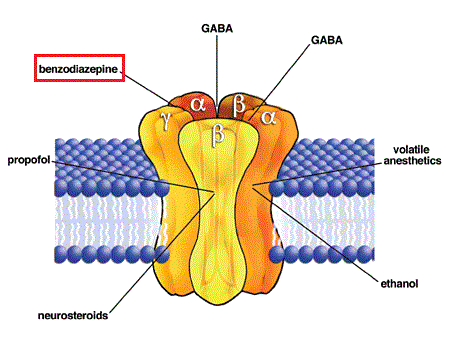 |
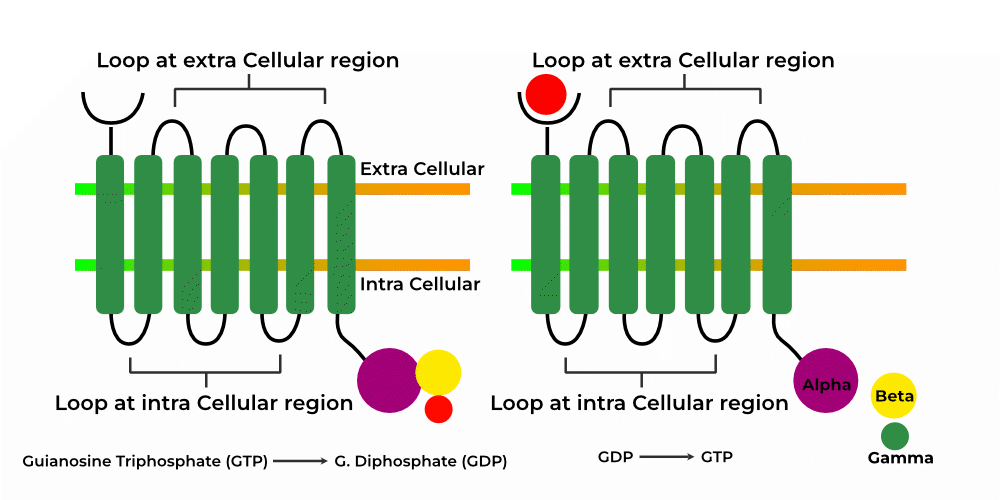
Abstract Gabapentin (GBP) was originally developed as a potential agonist for Gamma-Amino-Butyric-Acid (GABA) receptors, aiming to inhibit the activation of pain-signaling neurons. Contrary to initial expectations, it does not bind to GABA receptors. Instead, it exhibits several distinct pharmacological activities, including: (1) binding to the alpha-2-delta protein subunit of voltage-gated Gabapentin Gabapentin is similar in structure to γ-aminobutyric acid (GABA) but does not bind to GABAa or GABAb receptors. It binds to high-affinity gabapentin binding sites in the brain that possess the alpha-2-delta-1 subunit, which modulates the release of excitatory neurotransmitters. The gabapentinoid drugs do not bind significantly to other known drug receptors and so the α 2 δ VGCC subunit has been called the gabapentin receptor. [15][4] Recently, the same α 2 δ-1 protein has been found closely associated not with VGCCs but with other proteins such as presynaptic NMDA-type glutamate receptors, cell adhesion molecules The gabapentinoid drugs gabapentin and pregabalin are key front‐line therapies for various neuropathies of peripheral and central origin. Originally designed as analogs of GABA, the gabapentinoids bind to the α 2 δ‐1 and α 2 δ‐2 auxiliary subunits Gabapentin does not affect ligand binding at a wide variety of commonly-studied drug and neurotransmitter binding sites and voltage-activated ion channels including GABA, glutamate, and glycine receptors of several types (Taylor, 1995). The α2δ-1-NMDA Receptor Complex Is Critically Involved in Neuropathic Pain Development and Gabapentin Therapeutic Actions Gabapentinoids are first-line treatments for chronic pain but are associated with adverse side effects. A cryo-EM structure of gabapentin bound to its interaction site on the calcium channel α2δ Gabapentin antagonizes thrombospondin binding to alpha2delta-1 and powerfully inhibits excitatory synapse formation in vitro and in vivo. These findings identify alpha2delta-1 as a receptor involved in excitatory synapse formation and suggest that gabapentin may function therapeutically by blocking new synapse formation. Together with original binding data establishing the very low affinity of gabapentin for the GABA B receptor [4], these results suggest that the gabapentin effects are not directly mediated by the GABA B receptor, or at least the mechanism of action of gabapentin is quite different from that of other GABA B agonists. The data reveal a binding pocket in the Ca V α 2 δ-1 dCache1 domain that completely encapsulates gabapentin and define Ca V α 2 δ isoform sequence variations that explain the gabapentin binding selectivity of Ca V α 2 δ-1 and Ca V α 2 δ-2. Gabapentin has no activity at GABAA or GABAB receptors of GABA uptake carriers of brain. Gabapentin interacts with a high-affinity binding site in brain membranes, which has recently been identified as an auxiliary subunit of voltage-sensitive Ca2+ channels. However, the functional correlate of gabapentin binding is unclear and remains under study. Gabapentin works by showing a high affinity for binding sites throughout the brain corresponding to the presence of the voltage-gated calcium channels, especially α-2-δ-1, which seems to inhibit the release of excitatory neurotransmitters in the presynaptic area that participate in epileptogenesis. However, receptor binding studies have failed to demonstrate a direct binding site for gabapentin at the NMDA receptor [38–40]. The α 2 δ subunit of the voltage-dependent calcium channel is a binding site for gabapentin and the S -isomer of pregabolin (S - (+)-3-isobutylgaba) [4, 33, 37, 41]. Gabapentinoid drugs are widely used for pain, epilepsy and mental disorders. Chen et al. report the structure of a gabapentin-bound brain and heart calcium channel, revealing the gabapentin Gabapentin (GBP) was originally developed as a potential agonist for Gamma-Amino-Butyric-Acid (GABA) receptors, aiming to inhibit the activation of pain-signaling neurons. Contrary to initial expectations, it does not bind to GABA receptors. Instead, it exhibits several distinct pharmacological activities, including: (1) binding to the alpha-2-delta protein subunit of voltage-gated calcium However, gabapentin was shown to increase expression of δGABAA receptors, inhibitory tone in the cerebellum, and brain GABA concentration in patients, 3,4 while pregabalin enabled a larger neuronal calcium influx for facilitating neurotransmission. 2 These findings substantiate a GABAergic effect of gabapentin and pregabalin. Practice MCQs Q1. Gabapentin exerts its action by binding to: a. GABA-A receptor b. Sodium channels c. α2δ subunit of calcium channels d. NMDA receptor Q2. What is the effect of gabapentin on calcium influx? a. Increases Ca²⁺ influx b. No effect c. Reduces Ca²⁺ influx d. Blocks calcium reuptake Q3. Gabapentin is structurally similar to: a. Glycine b. GABA c. Glutamate d. Serotonin Q4 Pharmacodynamics Mechanisms of action Gabapentin and pregabalin do not bind to GABA receptors despite their structural similarity but have a high affinity for the α2δ-1 subunit of voltage-gated calcium channels (VGCCs). 19 VGCCs are composed of multiple subunits: α 1, β, γ and α 2 δ. The key residues involved in gabapentin interactions are also essential for the binding of l-Leu; however, the hydrogen-bonding network around these two ligands exhibits key differences. Gabapentin antagonizes thrombospondin binding to α2δ–1 and powerfully inhibits excitatory synapse formation in vitro and in vivo. These findings identify α2δ–1 as a receptor involved in excitatory synapse formation and suggest that gabapentin may function therapeutically by blocking new synapse formation. INTRODUCTION
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
12097-4/asset/b8e7d24e-0e2e-463d-967e-9e3ed3b43665/main.assets/gr1_lrg.jpg) |  |
 | |
 |  |
 |  |