Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 |  |
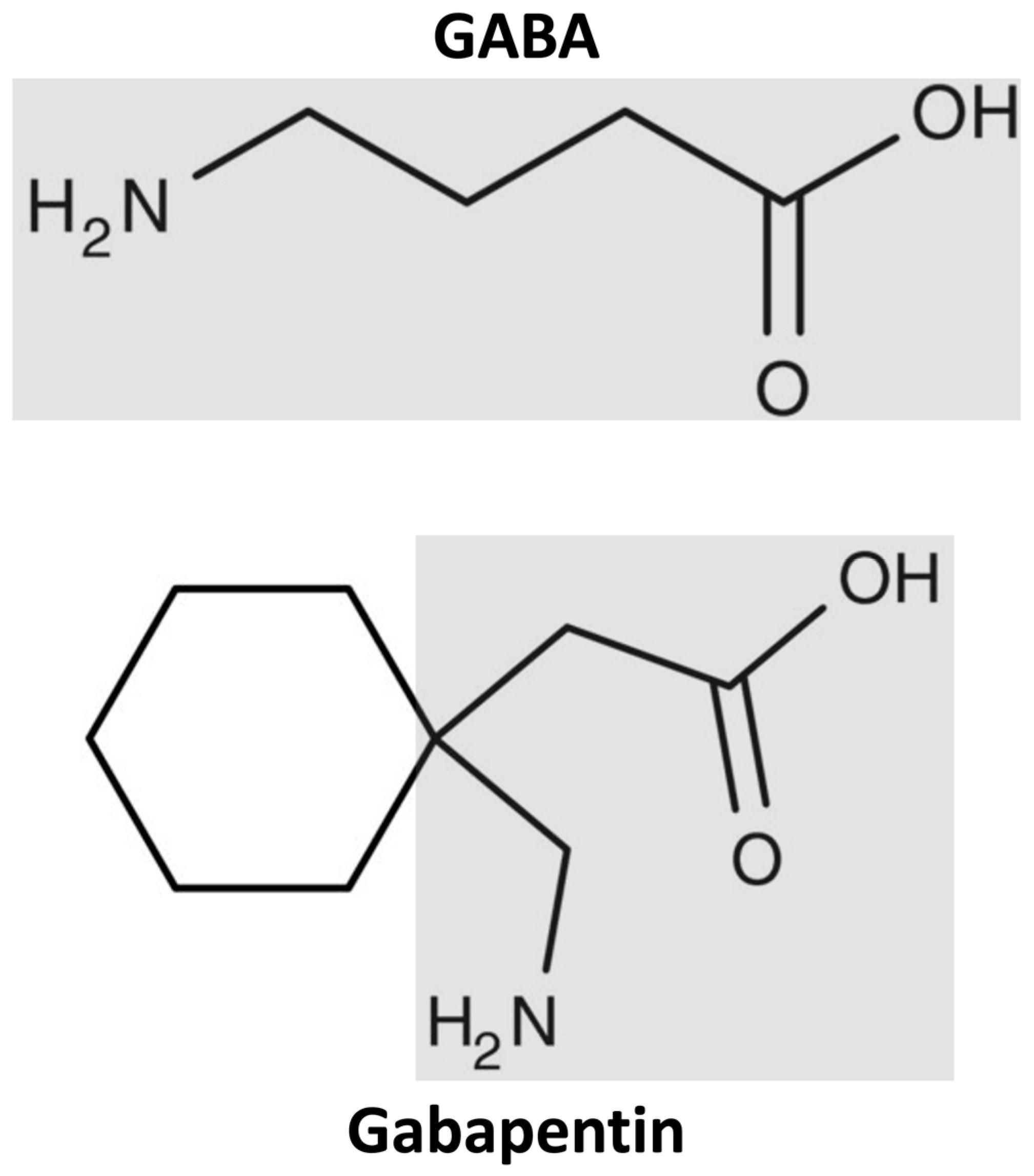
Treatment of Overdosage Gabapentin can be removed by hemodialysis. Although hemodialysis has not been performed in the few overdose cases reported, it may be indicated by the patient’s clinical state or in patients with significant renal impairment. Information on drug and health products authorized by Health Canada. Pr GABAPENTIN Gabapentin Capsules This leaflet is part III of a three-part "Product Monograph" published when GABAPENTIN was approved for sale in Canada and is designed specifically for Consumers. This leaflet is a summary and will not tell you everything about GABAPENTIN. Contact your doctor or pharmacist if you have any questions about the drug. Discontinuation of Treatment with GABAPENTIN: As with other anticonvulsant agents, abrupt withdrawal is not recommended because of the possibility of increased seizure frequency. There have been post-marketing reports of adverse events such as anxiety, insomnia, nausea, pain and sweating following abrupt discontinuation of treatment. (See 8.5 Post-Market Adverse Drug Reactions). When in the Gabapentin by Sanis Health Inc. is an anti-convulsant medication that inhibits the release of excitatory neurotransmitters, allowing for its use against pathologic neurotransmission such as that seen in neuropathic pain and seizure disorders. It has a wide therapeutic index, with doses in excess of 8000 mg/kg failing to cause a fatal reaction in rats. Such symptoms included agitation, disorientation and confusion after suddenly discontinuing gabapentin that resolved after restarting gabapentin. Most of these individuals had a history of poly-substance abuse or used gabapentin to relieve symptoms of withdrawal from other substances. Product name: GABAPENTIN Company name: SANIS HEALTH INC DIN: 02431297 Status: Cancelled Post Market Status date: 2025-03-24 Gabapentin reference guide for safe and effective use from the American Society of Health-System Pharmacists (AHFS DI). As Canada's trusted pharmacy, Rexall provides detailed drug factsheets for Gabapentin by Sanis Health Inc. with common uses, dosage instructions, side effects & drug interactions. This leaflet is part III of a three-part “Product Monograph” published when GABAPENTIN was approved for Product description Product name: GABAPENTIN Company name: SANIS HEALTH INC DIN: 02431289 Status: Marketed Status date: 2020-08-06 This document plus the full product monograph, prepared for health professionals, can be obtained by contacting Sanis Health Inc. at 1-866-236-4076 or quality@sanis.com. USP 35 Gabapentin RS, USP Gabapentin Related Compound A RS, and USP Gabapentin Related Compound B RS, respectively. Test solution—Use the Assay preparation. Standard solution—Dissolve a suitable quantity of USP Gabapentin Related Compound E RS in Diluent to obtain a solu-tion having a known concentration of 8.4 mg per mL. 1 NEURONTIN® (Gabapentin Capsules, 100 mg, 300 mg and 400 mg; Gabapentin Tablets, 600 mg and 800 mg), submission control 275525, Product Monograph, BGP Pharma ULC. It is not necessary to monitor gabapentin plasma concentrations in order to optimize GABAPENTIN therapy. Further, as there are no drug interactions with commonly used antiepileptic drugs, GABAPENTIN may be used in combination with these drugs without concern for alteration of plasma concentrations of either gabapentin or other antiepileptic drugs. DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. Pr GABAPENTIN Gabapentin Capsules, House Standard 100 mg, 300 mg, and 400 mg Antiepileptic Agent Sanis Health Inc. 1 President’s Choice Circle Brampton, Ontario MYLAN-GABAPENTIN (gabapentin) may be used in combination with other commonly used antiepileptic drugs without concern for alteration of the blood concentrations of gabapentin or other antiepileptic drugs. This leaflet is part III of a three-part "Product Monograph" published when GABAPENTIN was approved for sale in Canada and is designed specifically for Consumers. This leaflet is part III of a three-part “Product Monograph" published when TEVA-GABAPENTIN was approved for sale in Canada and is designed specifically for Consumers.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 |  |