Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |
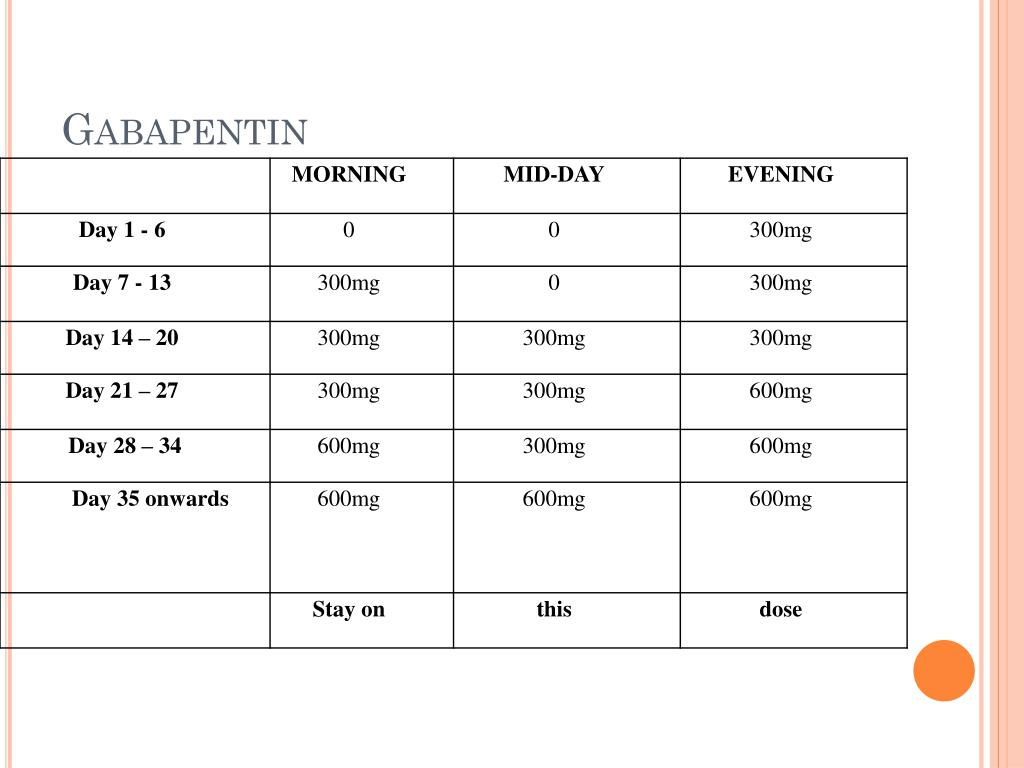
Key takeaways: Gabapentin (Neurontin) is FDA-approved to treat specific types of nerve pain and seizures. It’s also sometimes used to treat other health conditions. These include restless leg syndrome, anxiety, and alcohol withdrawal. Gabapentin isn’t a controlled substance according to the federal government. But several states have passed their own laws classifying gabapentin a schedule All you need to know about: Pregabalin and gabapentin reclassification Here we explain the prescribing and dispensing changes that will need to take place when pregabalin and gabapentin become Schedule 3 Controlled Drugs. RX DRUG SCHEDULING & MONITORING Prescription Drug Monitoring Programs (PDMPs) are electronic databases that collect information on the dispensing and prescribing of drugs within jurisdictions. PDMPs aim to assist patients in their quality of care by allowing prescribers and dispensers access to the patient’s controlled substance prescription medication history. This access to individual Re: Handling of gabapentin and pregabalin as Schedule 3 Controlled Drugs in health and justice commissioned services The purpose of this letter is to inform NHS England commissioned health and justice services and key stakeholders about the expectations for the handling of gabapentin and pregabalin as Schedule (Sch) 3 Controlled Drugs (CDs) from 1 April 2019. Now let’s simplify things Even though none of the schedule 3 controlled drugs need to be recorded in a CD register, we do advise you to record temazepam and buprenorphine in the CD register. Otherwise, what’s the point of keeping them in a CD cabinet if you then don’t count them? So what about pregabalin and gabapentin? The ACMD recommended that gabapentin and pregabalin be controlled as Class C drugs under the 1971 Act, and placed in Schedule 3 to the 2001 Regulations. Gabapentin is classified as a controlled substance in several states, including Alabama, Georgia, Kentucky, Tennessee, and Texas. These states have placed it under Schedule V, indicating a lower potential for abuse compared to higher schedules. Schedule 3 includes the barbiturates (except secobarbital, now Schedule 2), buprenorphine, gabapentin, mazindol, meprobamate, midazolam, pentazocine, phentermine, pregabalin, temazepam, and tramadol hydrochloride. They are subject to the special prescription requirements. Gabapentin is a prescription medication approved by the FDA for the treatment of neuropathic pain (postherpetic neuralgia) and seizure disorders. Why is gabapentin controlled in some states? Gabapentin is structurally and pharmacologically related to pregabalin (Lyrica, Lyrica CR), which is a Schedule V drug and controlled federally in all states. Individuals at the highest risk for abusing gabapentin include those with opioid abuse, mental illness, or previous history of prescription drug abuse. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. View gabapentin information, including dose, uses, side-effects, renal impairment, pregnancy, breast feeding, monitoring requirements and important safety information. Briefing Note From 1 April 2019, gabapentin and pregabalin will be reclassified as Schedule 3 controlled drugs under the Misuse of Drugs Regulations 2001, and Class C of the Misuse of Drugs Act 1971. Drug Schedules Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules depending upon the drug’s acceptable medical use and the drug’s abuse or dependency potential. The abuse rate is a determinate factor in the scheduling of the drug; for example, Schedule I drugs have a high potential for abuse and the potential to create Individuals at the highest risk for abusing gabapentin include those with opioid abuse, mental illness, or previous history of prescription drug abuse. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. We would like to show you a description here but the site won’t allow us. Dispensing and Supply February 2019 PSNC Briefing 010/19: Reclassification of gabapentin and pregabalin as CDs From 1st April 2019, amendments to the Misuse of Drugs Regulations 2001 and the Safe Custody Regulations 1973 come into force which mean that pregabalin and gabapentin will be reclassified as Schedule 3 Controlled Drugs (CDs). Introduction Gabapentin is chemically known as 2-[1-(aminomethyl) cyclohexaneacetic acid]. Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. FOR INFORMATION Gabapentin and pregabalin will be reclassified to Schedule 3 Controlled Drugs (CDs) (CD No Reg POMs). The table below summarises the changes that will apply from 1 April 2019. The Home Office has placed pregabalin and gabapentin to Schedule 3 of the Misuse of Drug Regulations 2001 from 1 April 2019. Regional Variation Gabapentin’s regulatory status varies by state. Some states classify it as a Schedule V controlled substance due to concerns about misuse and its involvement in the opioid crisis. Others do not schedule it but require mandatory reporting to state prescription drug monitoring programs (PDMPs) to track prescribing and dispensing.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |