Gallery
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
 |  |
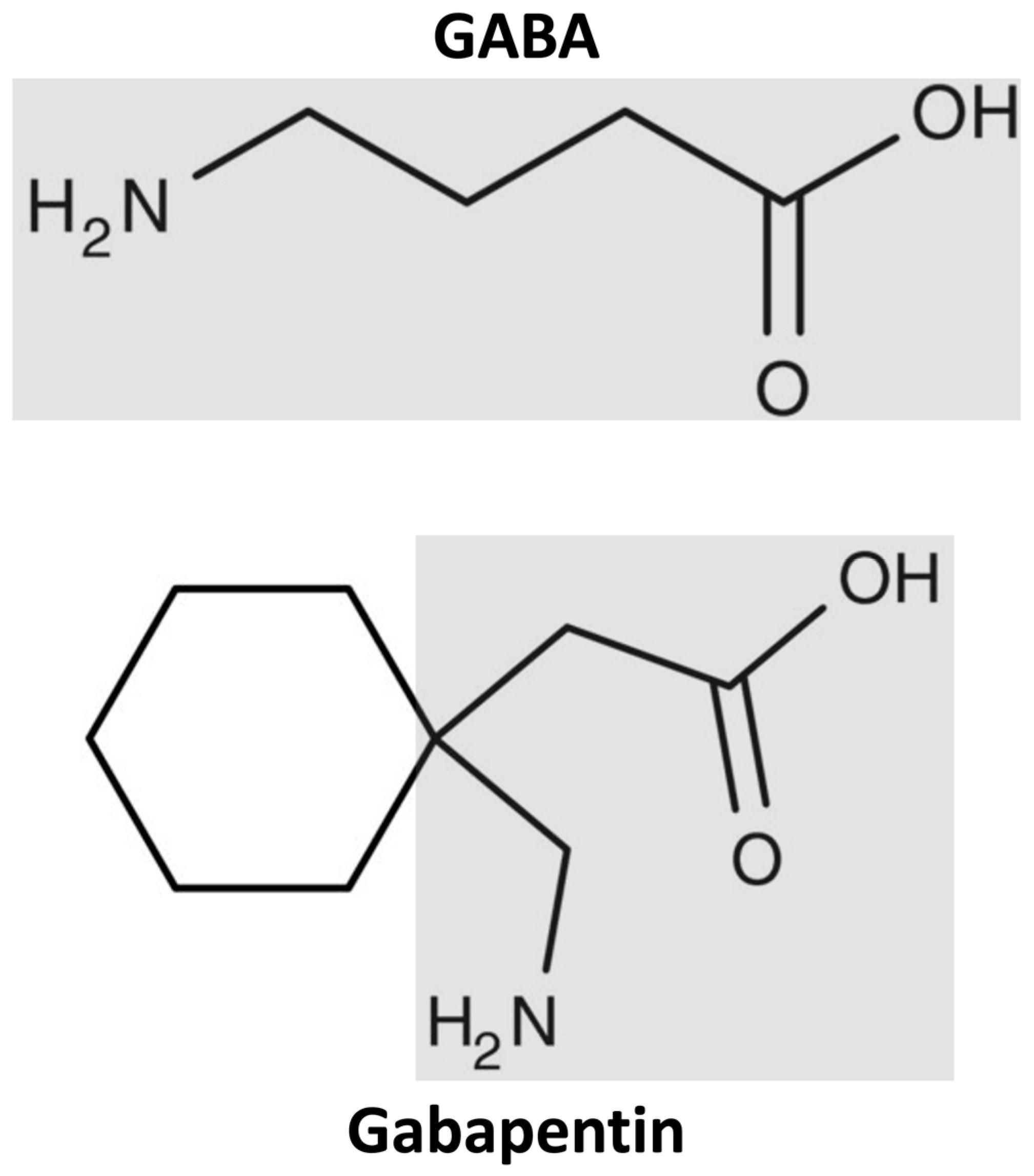
Gabapentin 100 mg capsules - Summary of Product Characteristics (SmPC) by Rivopharm UK Ltd Product Documentation View Summary of Product Characteristics (SmPC - Gabapentin Accord-UK 300mg Capsules) Last updated on this site: 25 Jan 2024 Gabapentin (Neurontin and associated names) has been approved in several Member States for the treatment of epileptic syndromes and several types of neuropathic pain. The precise mechanism of action of gabapentin is not known. Gabapentin is structurally related to the neurotransmitter GABA (gammaaminobutyric acid) and interacts with GABA synapses. On 2nd September 2004, Italy (Agencizia Treatment of peripheral neuropathic pain Gabapentin is indicated for the treatment of peripheral neuropathic pain such as painful diabetic neuropathy and post-herpetic neuralgia in adults. Gabapentin 300 mg capsules - Summary of Product Characteristics (SmPC) by Rivopharm UK Ltd Few population-based pregnancy outcome studies have been published to date. The two studies including the largest number of pregnancies exposed to gabapentin monotherapy did not report an increased risk of major congenital malformations (Hernández-Díaz S. et al., Källén B. et al.). A population-based study designed to assess the comparative risk of spontaneous abortions, terminations of Summaries of product characteristics form the basis of information for healthcare professionals on how to use the medicine safely and effectively. Abbreviated as SmPC. More information can be found under ' Product-information requirements ' and Guideline on summary of product characteristics' . The aim of this non-interventional study is to evaluate the use and safety of gabapentin in pregnancy using data on pregnancies identified from population-based registries in Denmark, Finland, Norway, and Sweden. To reduce confounding by indication or disease severity, in addition to the use of the AED-unexposed pregnancies as a comparator, this study also included, as active comparators Neurontin 100mg Hard Capsules - Summary of Product Characteristics (SmPC) by Upjohn UK Limited BACKGROUND INFORMATION Gabapentin (Neurontin and associated names) has been approved in several Member States for the treatment of epileptic syndromes and several types of neuropathic pain. The precise mechanism of action of gabapentin is not known. Gabapentin is structurally related to the neurotransmitter GABA (gamma-aminobutyric acid) and interacts with GABA synapses. On 2nd September 2004 European Medicines Agency alization in adults and adolescents aged 12 years. In addition, gabapentin is used for the treatment of peripheral neuropathic pain such as painful diabetic neuropathy and post-herpetic neuralgia in adults.1 Per current EU Mutual Recognition Procedure (MRP) Reference Member States (RMS) summary of product characteristics (SmPC), gabapentin f 400 mg and higher. Neurontin is available as 100 mg, 300 mg and 400 mg hard capsules and as 600 mg and 800 mg film‐coated tablets. The EMA guideline states that for drugs with a less than proportional increase in AUC with increasing dose over the therapeutic d Treatment of peripheral neuropathic pain Gabapentin is indicated for the treatment of peripheral neuropathic pain such as painful diabetic neuropathy and post-herpetic neuralgia in adults. Treatment of peripheral neuropathic pain Gabapentin is indicated for the treatment of peripheral neuropathic pain such as painful diabetic neuropathy and post-herpetic neuralgia in adults. A comprehensive description of the up-to-date indications and posology is given in the SmPC. Gabapentin 100 mg hard capsules - Summary of Product Characteristics (SmPC) by Morningside Healthcare Ltd Lyrica is a medicine that contains the active substance pregabalin. It is available as capsules (white: 25, 50 and 150 mg; white and orange: 75, 225 and 300 mg; orange: 100 mg; light orange: 200 mg) and as an oral solution (20 mg/ml). PRAC concluded that a causal association is established taking into account that a temporal relationship could be established with the start of gabapentin and the onset of agitation, positive de-challenge and positive re-challenge. Therefore the term has been included in section 4.8 of the SmPC. The European Medicines Agency has deferred the obligation to submit the results of studies with Qarziba in one or more subsets of the paediatric population in neuroblastoma (see section 4.2 for information on paediatric use). Grounds for the variation to the terms of the Marketing Authorisation(s) On the basis of the scientific conclusions for gabapentin the CMDh is of the opinion that the benefit-risk balance of the medicinal product(s) containing gabapentin is unchanged subject to the proposed changes to the product information.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
 |  |