Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
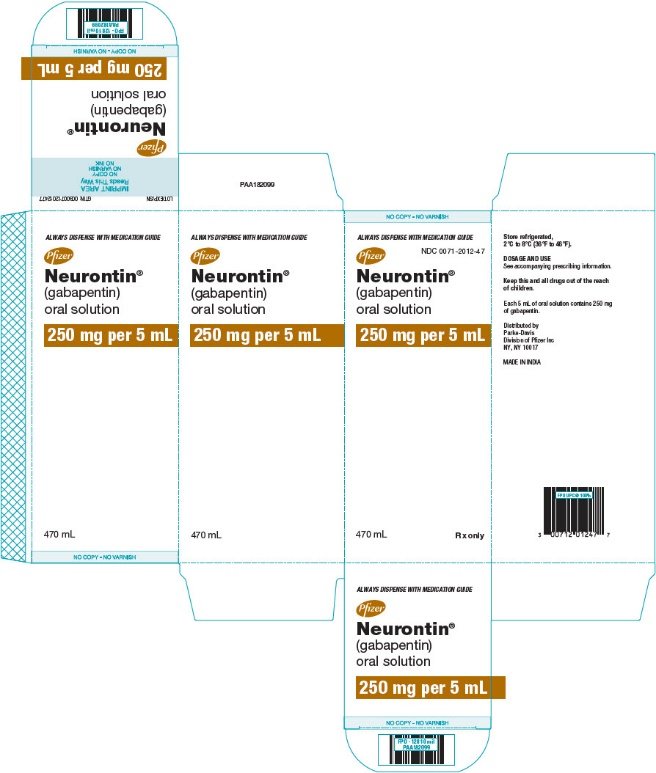 |  |
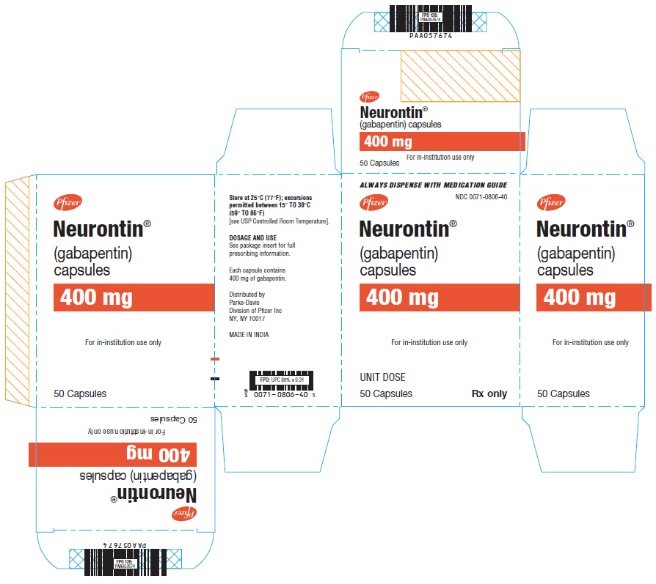
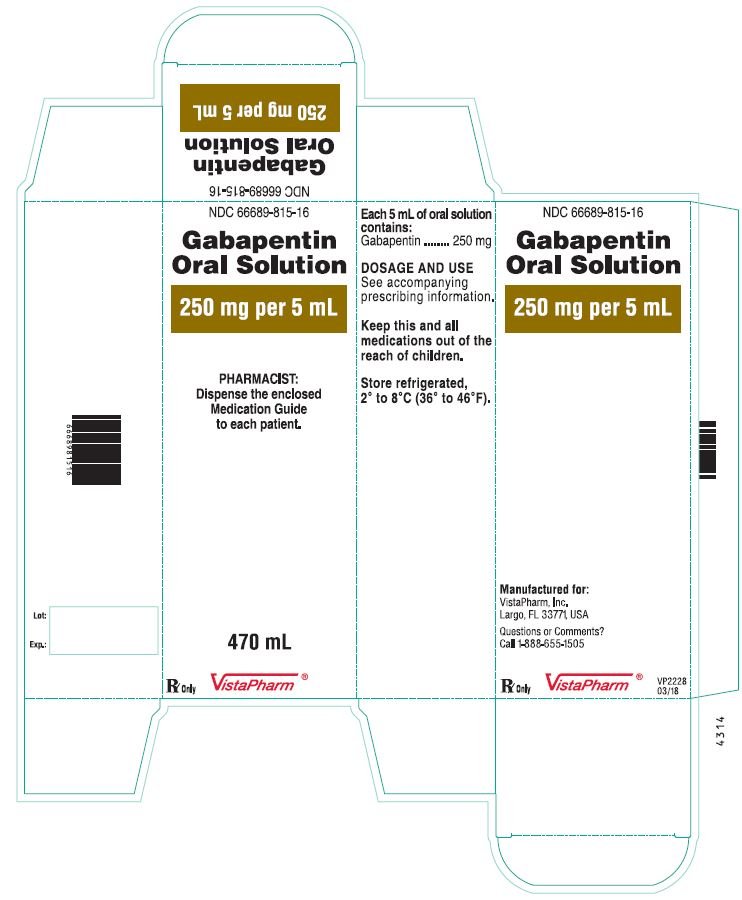
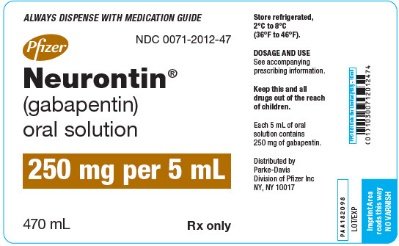
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GABAPENTIN ORAL SOLUTION safely and effectively. See full prescribing information for GABAPENTIN ORAL SOLUTION. Gabapentin may be administered as the oral solution, capsule, or tablet, or using combinations of these formulations. Dosages up to 50 mg/kg/day have been administered in a long-term clinical study. DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. 9.1 Controlled - Substance - Gabapentin oral solution contains gabapentin, which is not a controlled substance. 9.2 Abuse - Abuse is the intentional, non-therapeutic use of a drug, even once 10 OVERDOSAGE Signs of acute toxicity in animals included ataxia, labored breathing, ptosis, sedation, hypoactivity, or excitation. For more information about Gabapentin Oral Solution, medical inquiries, or to report side effects regarding Gabapentin Oral Solution, please call 1-800-541-4802. NEURONTIN oral solution contains 250 mg of gabapentin per 5 mL (50 mg per mL) and the following inactive ingredients: glycerin, xylitol, purified water, and artificial cool strawberry anise flavor. DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. Gabapentin Oral Solution is supplied as oral solution containing 250 mg/5 mL of gabapentin. The inactive ingredients are anise flavor natural and artificial, cooling agent flavor artificial, glycerin, purified water, strawberry flavor artificial and xylitol. The recommended maintenance dose of gabapentin oral solution in patients 3 to 4 years of age is 40 mg/kg/day, given in three divided doses. The recommended maintenance dose of gabapentin oral solution in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. Gabapentin Oral Solution package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. When prescribing gabapentin oral solution, carefully evaluate patients for a history of drug abuse and observe them for signs and symptoms of gabapentin misuse or abuse (e.g., self-dose escalation and drug-seeking behavior). BioComp Pharma, Inc.: Gabapentin oral solution is indicated for: Management of postherpetic neuralgia in adults. Adjunctive therapy in the treatment of partial onset seizures, with and without secondary NEURONTIN oral solution contains 250 mg of gabapentin per 5 mL (50 mg per mL) and the following inactive ingredients: glycerin, xylitol, purified water, and artificial cool strawberry anise flavor. Gabapentin Oral Solution - (gab-ah-PEN-tin) Read the Medication Guide before you start taking gabapentin and each time you get a refill. There may be new information. NEURONTIN oral solution contains 250 mg of gabapentin per 5 mL (50 mg per mL) and the following inactive ingredients: glycerin, xylitol, purified water, and artificial cool strawberry anise flavor. erious reaction. A healthcare provider should examine you to decide if you should continue t What is Gabapentin Oral Solution? Gabapentin Oral Solution is a prescription medicine used to treat: • Pain from damaged nerves (postherpetic pain) that follows healing of shingles (a painful rash that comes after a herpes zoster infection) in adults. HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GABAPENTIN ORAL SOLUTION safely and effectively. See full prescribing information for GABAPENTIN ORAL HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GABAPENTIN safely and effectively. See full prescribing information for GABAPENTIN. Quality Care Products, LLC: Gabapentin is indicated for: Management of postherpetic neuralgia in adults. Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in Delivers 6 mL - NDC 50383-351-06 - GABAPENTIN ORAL SOLUTION - 300 mg/6 mL - Each 6 mL contains 300 mg of gabapentin - FOR INSTITUTIONAL USE ONLY - Barcode: 3 5038335106 1 - USUSAL DOSAGE: See package insert
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |