Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 | |
 |  |
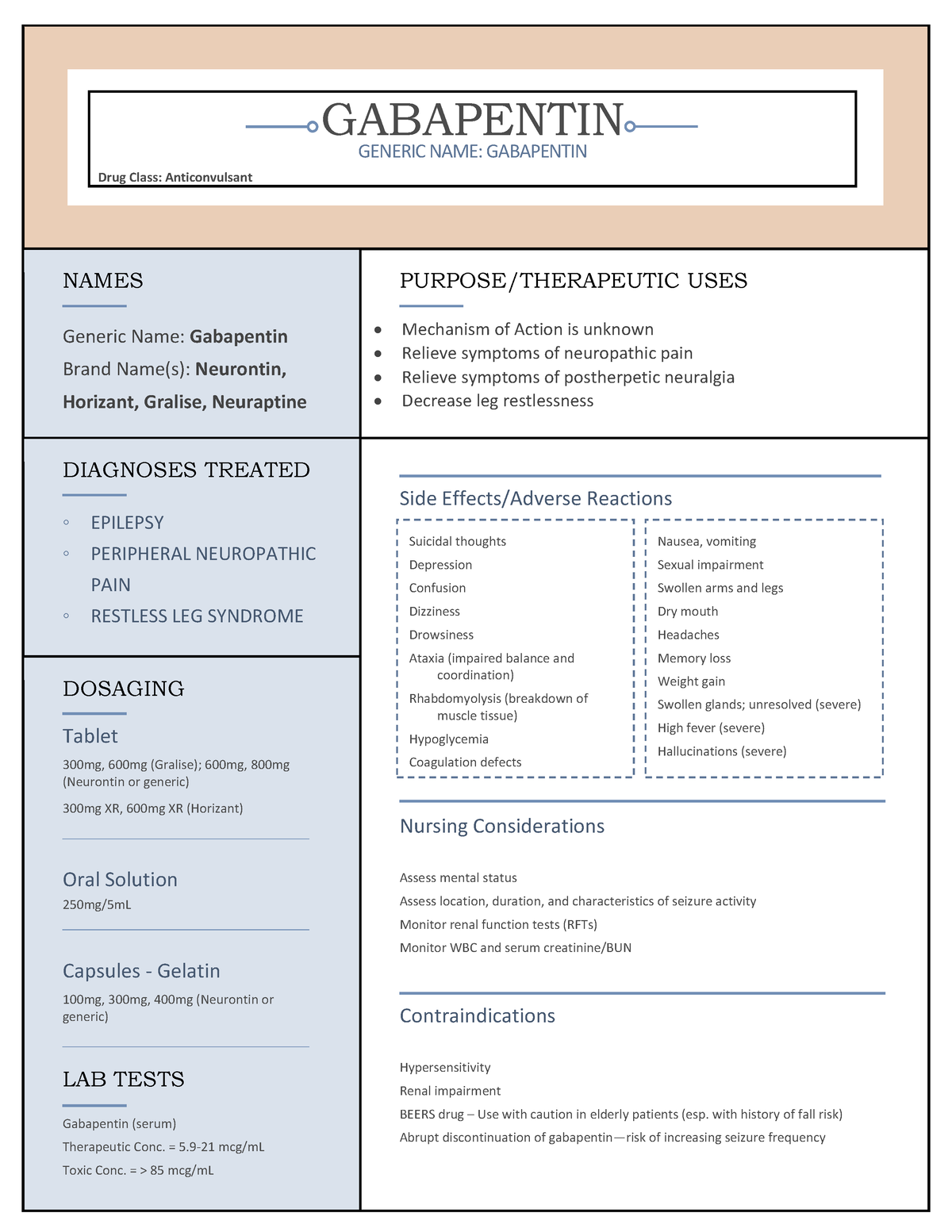
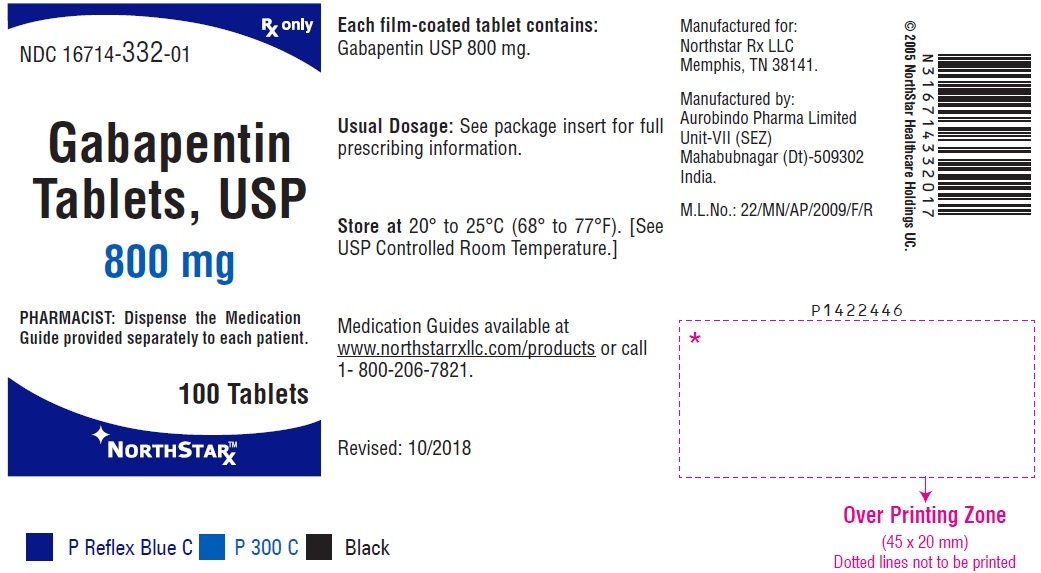
Precautions for Safe Handling Minimize dust generation and accumulation. If tablets or capsules are crushed and/or broken, avoid breathing dust and avoid contact with eyes, skin, and clothing. When handling, use appropriate personal protective equipment (see Section 8). Wash thoroughly after handling. Releases to the environment should be avoided. Review and implement appropriate technical and Details of the Supplier of the Safety Data Sheet Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-800-879-3477 Trade Name(s): Gabapentin Capsules, USP 100 mg, 300 mg and 400 mg. 1- acid; Neurontin Laboratory chemicals. Food, drug, pesticide or biocidal product use. Details of the supplier of the safety data sheet Material Name: Gabapentin Tablets (Neurontin) Trade Name: NEURONTIN; NORMATOL; GABAPENTIN Pfizer Chemical Family: Mixture Relevant Identified Uses of the Substance or Mixture and Uses Advised Against Intended Use: Pharmaceutical product used as anticonvulsant SAFETY DATA SHEET Date: 08/14/2024 Generic Name: Gabapentin Tablets, USP 600 and 800 mg. Microsoft Word - Gabapentine tabs-SDSSAFETY DATA SHEET Material Name: Gabapentin Capsules (100 mg, 300 mg and 400 mg) Trade Name: Chemical Family: Intended Use: Neurontin® Mixture Pharmaceutical product used as anticonvulsant Material Name: Gabapentin Tablets (Neurontin) Trade Name: Chemical Family: Intended Use: Neurontin® Mixture Pharmaceutical product used as anticonvulsant Gabapentin Tablets (Neurontin) MSDS | The new SDS required by OSHA are being added daily to check for a newer version of a safety data sheet search our free msds online database. Dose and Administration Gabapentin tablets are given orally with or without food. Patients should be informed that, should they break the scored 600 or 800 mg tablet in order to administer a half-tablet, they should take the unused half-tablet as the next dose. Half-tablets not used within several days of breaking the scored tablet should be discarded. If gabapentin tablets dose is reduced Carcinogenesis: Gabapentin was administered orally to mice and rats in 2-year carcinogenicity studies. No evidence of drug-related carcinogenicity was observed in mice treated at doses up to 2000 mg/kg/day. At 2000 mg/kg, the plasma gabapentin exposure (AUC) in mice is approximately 2 times that in humans at the MRHD of 3600 mg/day. In rats, increases in the incidence of pancreatic acinar cell EMERGENCY OVERVIEW Caution Statement: Each Gabapentin Tablets intended for oral administration contains Gabapentin and excipients generally considered to be non-toxic and non-hazardous in small quantities and under conditions of normal occupational exposure. Generic Name: Gabapentin Capsules, USP 100, 300 and 400 mg. Product Info Dosage FormTablet TE CodeAB2 Brand ReferenceGralise Therapeutic CategoryAnticonvulsants PronunciationGA-ba-PEN-tin Inactive Ingredients300 mg tablet: copovidone, hypromellose, lecithin (soya), magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyethylene oxide, polyvinyl alcohol-partially hydrolysed, talc and titanium dioxide. 600 mg tablet: copovidone Persistence and degradability AEROBIC: The anticonvulsant drug Gabapentin (GAB) and photolytic mixtures were submitted to the Closed Bottle Test (CBT; OECD 301 D) to assess biodegradability. The information and recommendations in this safety data sheet are, to the best of our knowledge, accurate as of the date of issue. Nothing herein shall be deemed to create any warranty, express or implied. EMERGENCY OVERVIEW Caution Statement: Each Gabapentin Tablets intended for oral administration contains Gabapentin and excipients generally considered to be non-toxic and non-hazardous in small quantities and under conditions of normal occupational exposure. The intent of this safety data sheet (SDS) is to provide safety information for occupational handling of this product. This information may not be relevant for medical use of this product. Patients/ consumers should consult the package insert/ product label/ physician/ pharmacist for information regarding the usage of this product. For information on the ingredients used in this product, refer Disclaimer The information provided on this SDS is correct to the best of our knowledge, information and belief at the date of its publication. The information given is designed only as a guide for safe handling, use, processing, storage, transportation, and disposal of the designated material and is not to be considered as a warranty or
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 | |
 |  |