Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
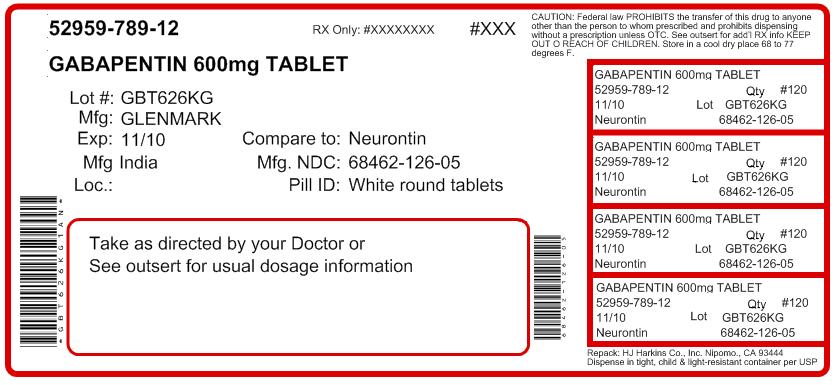
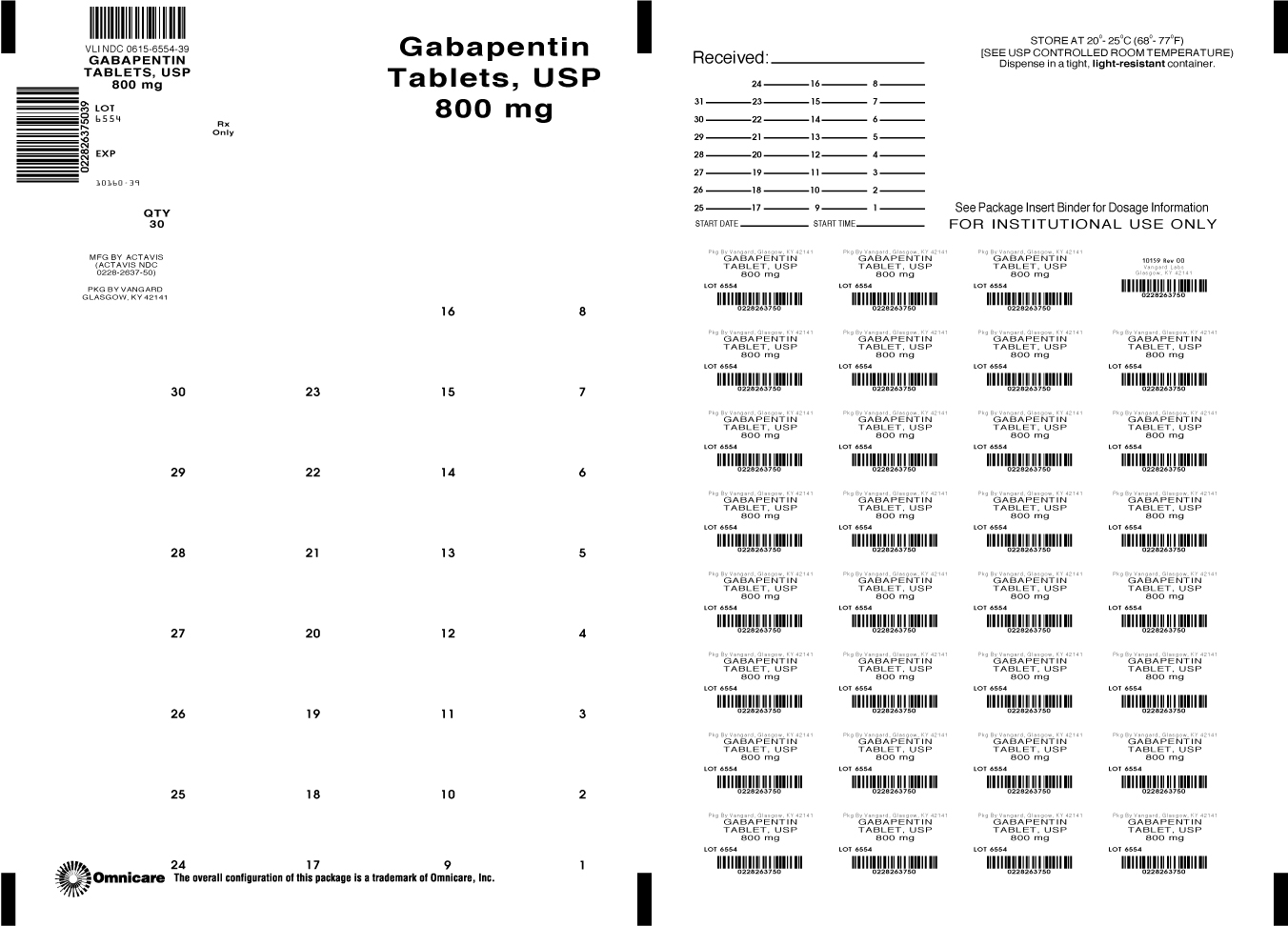
Gabapentin Tablets USP Generic Version of Neurontin ® Tablets Therapeutic Class: Anti-Convulsant TE Code: AB Test specimen—Grind at least 20 Tablets to a fine powder. Use an amount of powder, equivalent to about 2 mg of gabapentin, and about 200 mg of dry potassium bromide. Gabapentin Tablets contain NLT 90.0% and NMT 110.0% of the labeled amount of gabapentin (C 9 H 17 NO 2). United States Pharmacopeia (). USP Monographs, Gabapentin Tablets. . Rockville, MD: United States Pharmacopeia. Gabapentin tablets are indicated for: • Management of postherpetic neuralgia in adults - • Adjunctive therapy in the treatment of partial onset seizures, with and without secondary What is gabapentin? Gabapentin is a prescription medicine used to treat: • Pain from damaged nerves (postherpetic pain) that follows healing of shingles (a painful rash that comes after a herpes zoster infection) in adults. • Partial seizures when taken together with other medicines in adults and children 3 years of age and older with seizures. DEFINITION Gabapentin contains NLT 98.0% and NMT 102.0% of gabapentin (C 9 H 17 NO 2), calculated on the anhydrous basis. Gabapentin tablets USP are supplied as oval shaped, film-coated, biconvex scored tablets containing 600 mg and 800 mg of gabapentin USP. The inactive ingredients for the tablets are corn starch, copovidone, poloxamer 407, magnesium stearate, polyethylene glycol, talc, hypromellose, titanium dioxide, macrogol, polysorbate 80 and purified water. Gabapentin Tablets contain NLT 90.0% and NMT 110.0% of the labeled amount of gabapentin (C 9 H 17 NO 2). United States Pharmacopeia (2025). USP Monographs, Gabapentin Tablets. USP-NF. Rockville, MD: United States Pharmacopeia. For more information about gabapentin tablets and for medical inquiries or to report side effects regarding gabapentin tablets, please call 1-800-818-4555. What are the ingredients in gabapentin tablets? Gabapentin tablets, USP are indicated as adjunctive therapy in the treatment of partial seizures with and without secondary generalization in patients over 12 years of age with epilepsy. Dosage: Tablet, Strength: 800 mg, Brand name equivalent: Generic of Neurontin® Tablets The starting dose is 300 mg three times a day. The recommended maintenance dose of gabapentin tablets is 300 mg to 600 mg three times a day. Dosages up to 2400 mg/day have been well tolerated in long-term clinical studies. Doses of 3600 mg/day have also been administered to a small number of patients for a relatively short duration, and have been well tolerated. Administer gabapentin tablets Gabapentin Tablets, USP 600 mg: White colored film coated, Modified Capsule shaped, biconvex tablets debossed with '1' on the left side of the bisect and '2' on the right side of the bisect on 4 CONTRAINDICATIONS Gabapentin Tablets, USP are contraindicated in patients who have demonstrated hypersensitivity to the drug or its ingredients. Gabapentin tablets USP are supplied as oval shaped, film-coated, biconvex scored tablets containing 600 mg and 800 mg of gabapentin USP. The inactive ingredients for the tablets are corn starch, copovidone, poloxamer 407, magnesium stearate, polyethylene glycol, hypromellose, titanium dioxide, talc, macrogol, polysorbate 80 and purified water. Each gabapentin tablet contains 600 mg or 800 mg of gabapentin, USP and the following inactive ingredients: poloxamer 407, mannitol, magnesium stearate, hydroxypropyl cellulose, talc, copovidone, crospovidone, colloidal silicon dioxide and opadry white (03F180003). United States Pharmacopeia (). USP Monographs, Gabapentin Capsules. USP-NF. Rockville, MD: United States Pharmacopeia. View the USP Certificate or Product Information Sheet in the table above to view additional product details, including available label text and storage information. Each film-coated tablet contains 600 mg of gabapentin, USP. See package insert for full prescribing information. [See USP Controlled Room Temperature]. Dispense in tight (USP), child-resistant containers. Rev. 09/2017. PHARMACIST: Dispense the Medication Guide provided separately to each patient. DESCRIPTION Gabapentin tablets, USP are supplied as film-coated tablets containing 600 mg and 800 mg of gabapentin USP. The inactive ingredients for the tablets are copovidone, corn starch, crospovidone, hydroxypropyl cellulose, magnesium stearate, microcrystalline cellulose, and talc. Gabapentin tablets are a prescription medicine used to treat: • Pain from damaged nerves (postherpetic pain) that follows healing of shingles (a pain. ul rash that comes after a herpes zoster infection) in adults. • Partial seizures when taken together with other medicines. in adults and children 3 years of age a.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |