Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
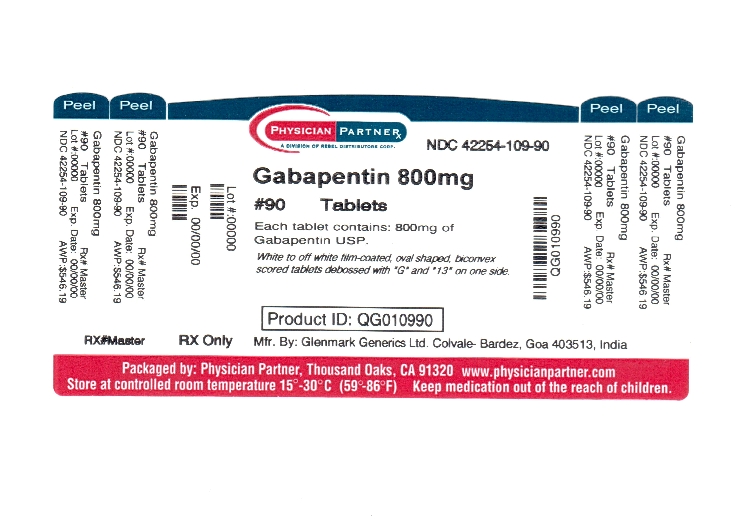
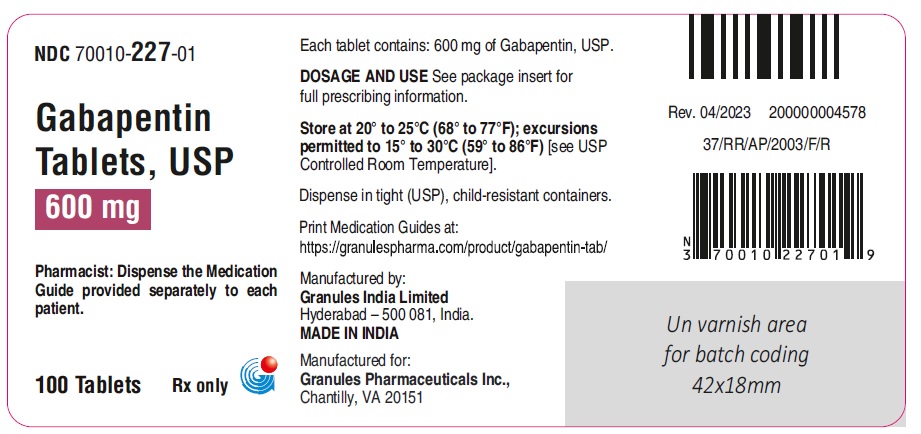
The Gabapentin Tablets Revision Bulletin supersedes the monograph in USP 32–NF 27 until it is printed in the USP 33–NF 28 First Supplement which will be released 1 February 2010 and becomes official 1 August 2010. Should you have any questions, please contact Margareth Marques, Ph.D. (301-816-8106 or mrm@usp.org). United States Pharmacopeia (). USP Monographs, Gabapentin. USP-NF. Rockville, MD: United States Pharmacopeia. United States Pharmacopeia (). USP Monographs, Gabapentin Tablets. . Rockville, MD: United States Pharmacopeia. Each gabapentin tablet contains 600 mg or 800 mg of gabapentin, USP and the following inactive ingredients: poloxamer 407, mannitol, magnesium stearate, hydroxypropyl cellulose, talc, copovidone, crospovidone, colloidal silicon dioxide and opadry white (03F180003). United States Pharmacopeia (2025). USP Monographs, Gabapentin Tablets. USP-NF. Rockville, MD: United States Pharmacopeia. PRODUCT MONOGRAPH Pr MYLAN-GABAPENTIN (Gabapentin Capsules) 100 mg, 300 mg, and 400 mg (Gabapentin Tablets, USP) 600 mg and 800 mg Gabapentin Tablets » Gabapentin Tablets contain not less than 90.0 percent and not more than 110.0 percent of the labeled amount of gabapentin (C9H17NO2). l-closed containers. Store at control USP Gabapentin RS. USP Gabapentin R As with any CNS active drug, physicians should carefully evaluate patients for a history of drug abuse and follow such patients closely, observing them for signs of abuse or misuse of gabapentin (e.g. development of tolerance, self-dose escalation, and drug-seeking behavior). Official Monographs / Gabapentin 3297 . Gabapentin Capsules » Gabapentin Capsules contain not less than 90.0 percent and not more than 110.0 per cent of the labeled amount of gabapentin (C 9H17NO2). Packaging and storage—Preserve in well-closed containers. Store at controlled room temperature. This leaflet is part III of a three-part “Product Monograph" published when Gabapentin Capsules USP and Gabapentin Tablets USP was approved for sale in Canada and is designed specifically for Consumers. USP 35 Gabapentin RS, USP Gabapentin Related Compound A RS, and USP Gabapentin Related Compound B RS, respectively. Test solution—Use the Assay preparation. Standard solution—Dissolve a suitable quantity of USP Gabapentin Related Compound E RS in Diluent to obtain a solu-tion having a known concentration of 8.4 mg per mL. If therapy is discontinued, dosage reduced, or an alternative drug substituted, perform such changes gradually over ≥1 week. Gabapentin enacarbil extended-release tablets: Initially, 600 mg once daily in the morning for 3 days, then increase to recommended maintenance dosage of 600 mg twice daily. DESCRIPTION Gabapentin tablets, USP are supplied as elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin USP. Standard solution Dissolve accurately weighed quantities of USP Gabapentin RS and USP Gabapentin Related Compound A RS in Diluent to obtain a solution having a known concentration of about 0.04 mg of each per mL. Test solution Remove and weigh the contents of not fewer than 20 Capsules. Diluent, Buffer solution, Mobile phase, and Chromatographic system— Proceed as directed in the Assay. Impurities solution— Dissolve suitable quantities of USP Gabapentin Related Compound A RS and USP Gabapentin Related Compound B RS in methanol to obtain a solution containing about 1.4 mg per mL and 0.84 mg per mL, respectively. A comparative, two-way, single-dose, bioavailability study was performed under fed conditions on TEVA-GABAPENTIN (gabapentin) 800 mg tablets and Neurontin® 800 mg tablets by Parke-Davis division of Warner-Lambert Canada. CONTRAINDICATIONS Gabapentin Capsules USP and Gabapentin Tablets USP (gabapentin) are contraindicated in patients who are hypersensitive to this drug or to any ingredient in the formulation, including any non-medicinal ingredients, or component of the container. For a complete listing, see 6 DOSAGE FORMS, STRENGTHS, MEDICATION GUIDE GABAPENTIN CAPSULE, USP GABAPENTIN TABLETS, USP (GAB-a-PEN-tin) ld know about gabapen Do not stop taking gabapentin without first talking to your healthcare provider. Submission Control No.: 281838 GLN-Gabapentin (Gabapentin Tablets USP, 600 mg and 800 mg) Page 1 of 36 United States Pharmacopeia (). USP Monographs, Gabapentin Capsules. USP-NF. Rockville, MD: United States Pharmacopeia.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |