Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
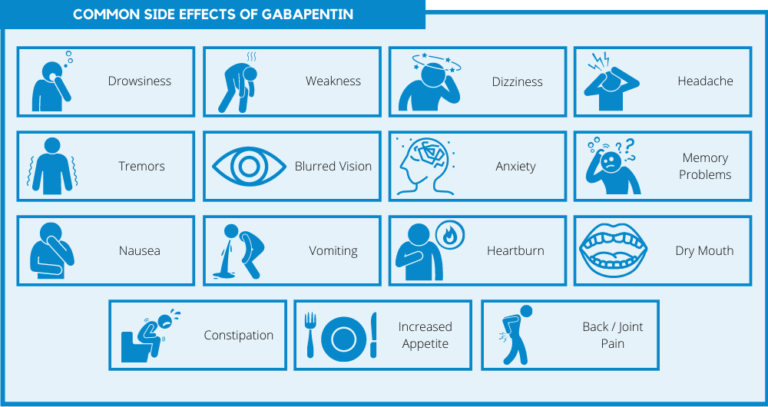
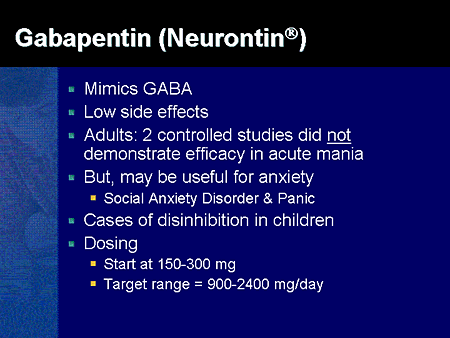
Gabapentin is an anti-epileptic drug, also called an anticonvulsant. It is used to treat some types of seizures and nerve pain caused by shingles. FDA is requiring new warnings about risk of respiratory depression in patients who use gabapentanoids with opioids or drugs that depress the nervous system 2.1 Dosage for Postherpetic Neuralgia In adults with postherpetic neuralgia, NEURONTIN may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). The dose can subsequently be titrated up as needed for pain relief to a dose of 1800 mg/day (600 mg three times a day). In clinical studies, efficacy was Gabapentin and pregabalin are FDA-approved for a variety of uses include fibromyalgia and restless legs syndrome. Gabapentin was first approved in 1993 and pregabalin was When prescribing gabapentin carefully evaluate patients for a history of drug abuse and observe them for signs and symptoms of gabapentin misuse or abuse (e.g., development of tolerance, self-dose escalation, and drug-seeking behavior). The FDA recently issued strong gabapentin warnings to doctors. It should not be prescribed with opioids if patients have respiratory problems like COPD. Gabapentin is approved to prevent and control partial seizures, relieve postherpetic neuralgia after shingles and moderate-to-severe restless legs syndrome. Learn what side effects to watch for, drugs to avoid while taking gabapentin, how to take gabapentin and other important questions and answers. Gabapentin is available in both branded and generic forms. The FDA is warning of serious, life-threatening respiratory problems associated with gabapentin. FDA warns about serious breathing problems with seizure and nerve pain medicines gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica CR) FDA Drug Safety Podcast During the controlled epilepsy trials in patients older than 12 years of age receiving doses of NEURONTIN up to 1800 mg daily, somnolence, dizziness, and ataxia were reported at a greater rate in patients receiving NEURONTIN compared to placebo: i.e., 19% in drug versus 9% in placebo for somnolence, 17% in drug versus 7% in placebo for The FDA has issued a drug safety warning for gabapentin and pregabalin, including Gralise and Lyrica, after a review of data suggested that use of the drugs may result in serious breathing difficulties in patients who have respiratory risk factors. The FDA recently released a warning for the medications, gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica CR). The FDA warned that serious breathing difficulties may occur in patients using these medications who have respiratory risk factors. Advise the patient to read the FDA-approved patient labeling (Medication Guide). Administration Information - Inform patients that gabapentin capsules are taken orally with or without What Clinicians Need to Know About New FDA Respiratory Warnings on Gabapentin and Pregabalin Products New warnings link this drug class to respiratory depression and abuse potential. WARNINGS AND PRECAUTIONS 5.1 Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity 5.2 Anaphylaxis and Angioedema 5.3 Effects on Driving and Operating Heavy Machinery 5.4 Somnolence/Sedation and Dizziness 5.5 Withdrawal Precipitated Seizure, Status Epilepticus 5.6 Suicidal Behavior and Ideation 5.7 Neuropsychiatric Adverse Reactions (Pediatric Patients 3 to See full prescribing information for NEURONTIN. NEURONTIN® (gabapentin) capsules, for oral use NEURONTIN® (gabapentin) tablets, for oral use NEURONTIN® (gabapentin) oral solution Initial U.S. Approval: 1993 ---------------------------RECENT MAJOR CHANGES -------------------------- Warnings and Precautions, Respiratory Depression (5.7) 4/2020 ISSUE: FDA is warning that serious breathing difficulties may occur in patients using gabapentin (Neurontin, Gralise, Horizant) or pregabalin (Lyrica, Lyrica CR) who have respiratory risk factors. In 2019 the FDA issued a warning about the potential risks of respiratory depression in patients taking gabapentin or pregabalin in combination with central nervous system (CNS) depressants such as opioids, antidepressants, and benzodiazepines. Medications affected by this warning include gabapentin (brand names: Neurontin and Gralise), gabapentin enacarbil (a gabapentin pro-drug under the brand name Horizant), pregabalin (brand names: Lyrica and Lyrica CR) and generic versions of gabapentinoids. The U.S. Food and Drug Administration is warning that serious breathing problems can occur in patients who use gabapentin or pregabalin with opioids or other drugs that depress the central nervous system. The elderly and patients with lung problems are at higher risk when they use the drugs, according to an FDA drug safety communication.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |