Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
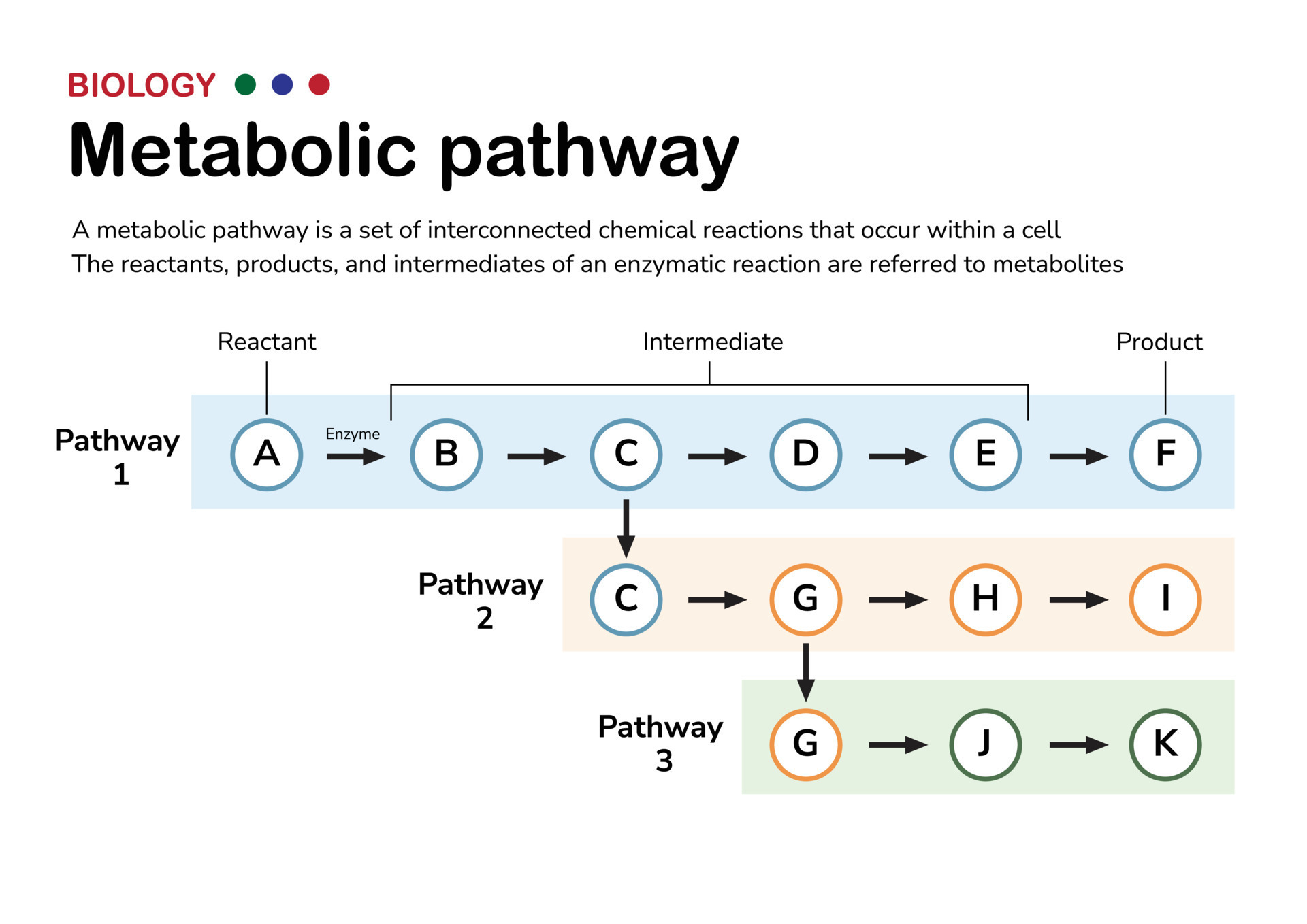
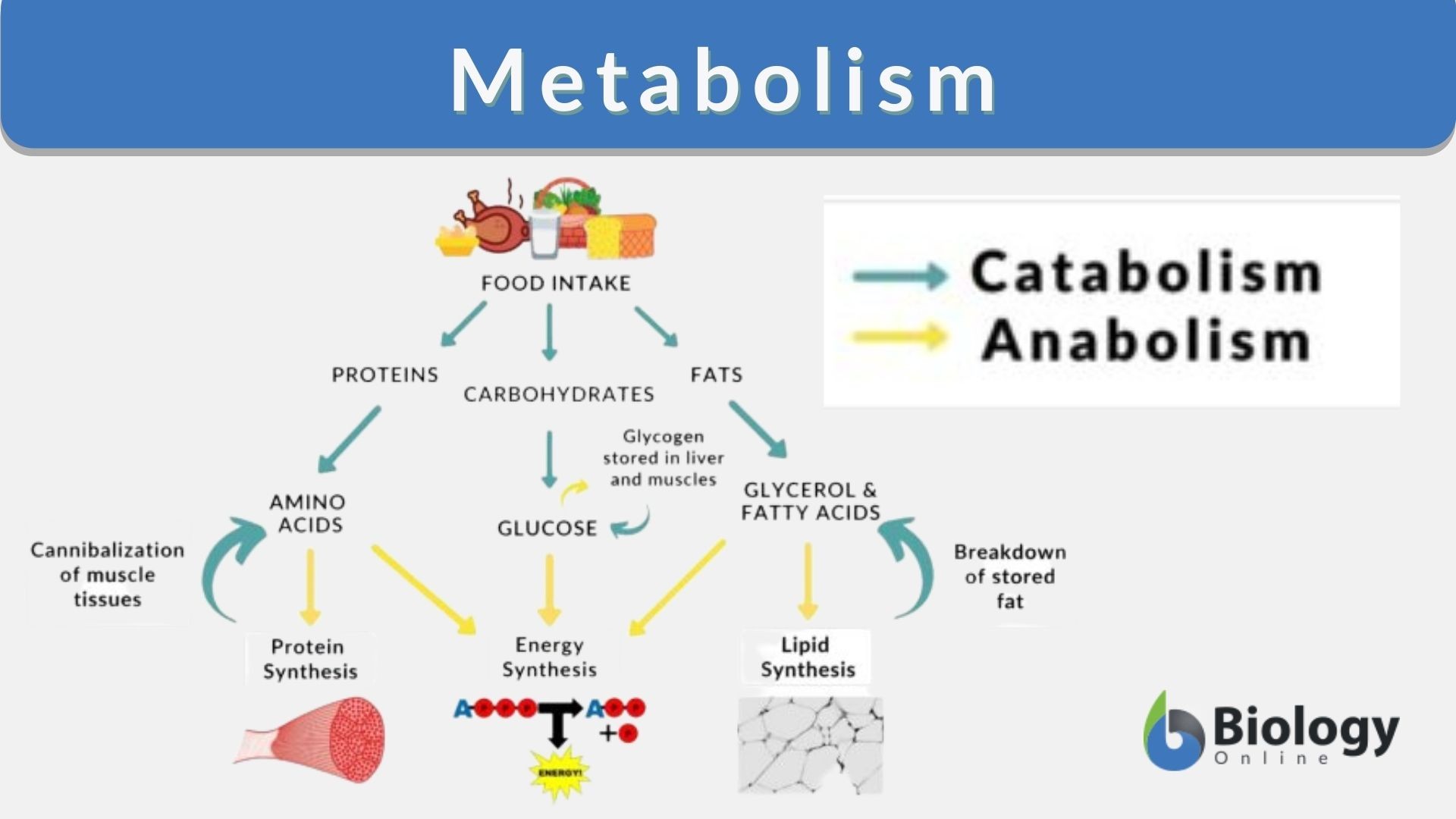
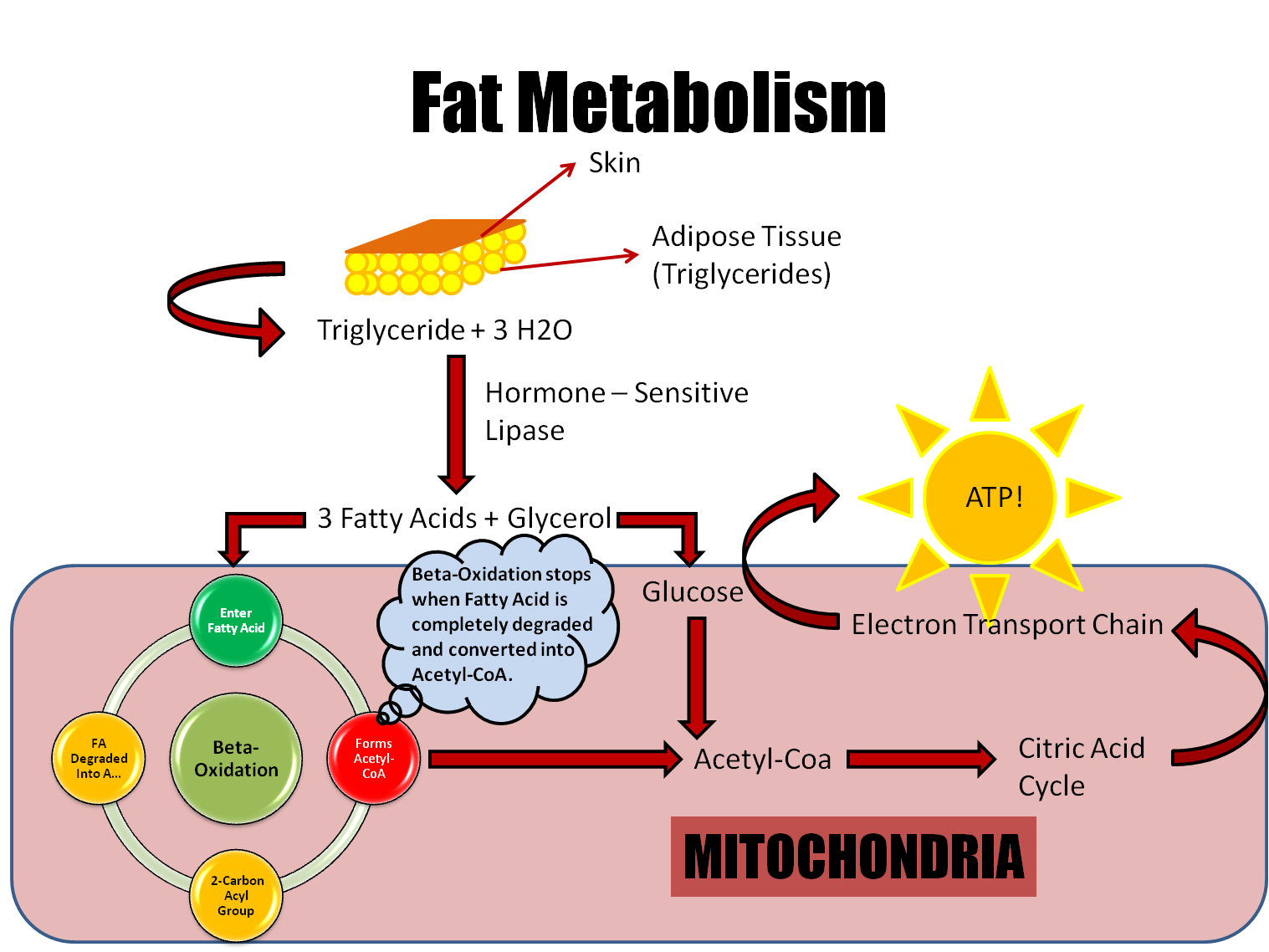
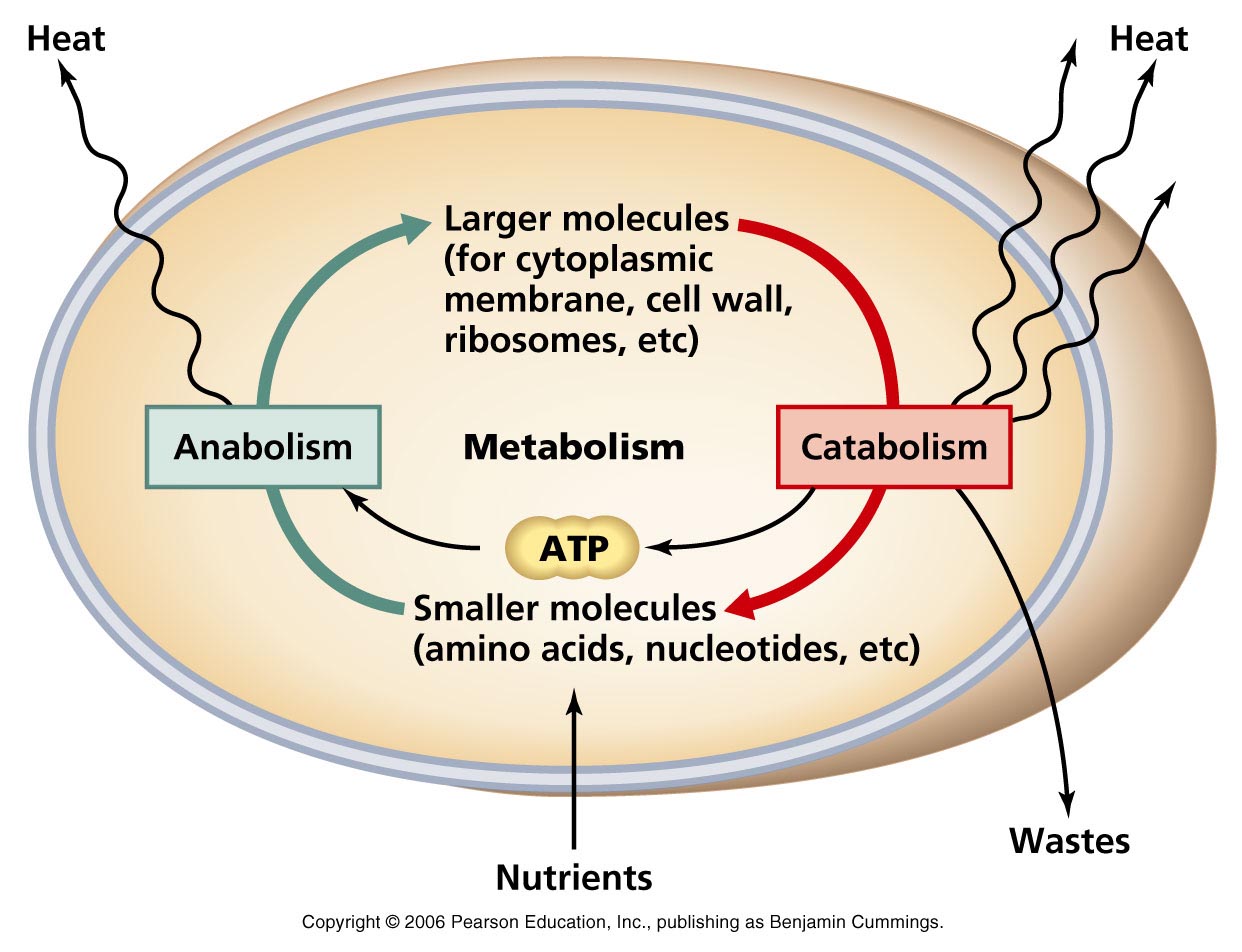
This paper describes the pharmacokinetic studies of 1- (aminomethyl)-cyclohexane acetic acid (gabapentin, Gö 3450, CI-945) conducted with the 14C-labelled substance following intravenous and intragastric administration to rats and dogs and oral administration to humans. The metabolism of gabapentin has not been studied in cats, but pharmacokinetics demonstrates faster elimination than in humans, with similar implications for dose intervals as in dogs. 3 A lack of hepatic metabolism makes gabapentin an attractive option for patients on multiple antiepileptic drugs and patients with impaired hepatic function. Although gabapentin is relatively well tolerated, patients should be counseled on and monitored for CNS-related adverse effects, including somnolence, dizziness, ataxia, and asthenia. Pregabalin and gabapentin are members of a unique class of compounds characterized by high affinity binding to the alpha-2-delta (α2δ) protein in the CNS. Both have been shown to be effective as adjunctive therapy for partial seizures and neuropathic pain disorders. Clinical pharmacology studies conducted to characterize the pharmacokinetics, bioavailability, and drug-drug interaction Gabapentin reference guide for safe and effective use from the American Society of Health-System Pharmacists (AHFS DI). Pharmacodynamics Mechanisms of action Gabapentin and pregabalin do not bind to GABA receptors despite their structural similarity but have a high affinity for the α2δ-1 subunit of voltage-gated cal-cium channels (VGCCs).19 VGCCs are composed of multiple subunits: α1, β, γ and α2δ. Gabapentin is a new antiepileptic drug (AED) with an attractive pharmacokinetic profile. It is absorbed by an active and saturable transport system, and has a high volume of distribution. Gabapentin is not bound to plasma proteins, does not induce hepatic enzymes and is not metabolized. At steady st Gabapentin (“Neurontin”) has been shown in extensive preclinical and clinical studies to be an effective anticonvulsant drug, which appears to have novel mechanisms of action. Although designed as a GABA analogue it is clearly not GABAmimetic, although Summary: This paper describes the pharmacokinetic studies of 1-(aminomethyl)-cyclohexane acetic acid (gabapentin, Go 3450, CI-945) conducted with the '4C-labelled substance fal-lowing intravenous and intragastric administration to rats and dogs and oral administration to humans. In adults, thyroid hormone helps to maintain brain function, food metabolism, and body temperature, among other effects. The symptoms of thyroid deficiency relieved by levothyroxine include slow speech, lack of energy, weight gain, hair loss, dry thick skin and unusual sensitivity to cold. Gabapentin | Deranged PhysiologyGabapentin Gabapentin is not protein-bound. A high volume of distribution indicates greater concentration in tissue than in plasma. It is not metabolized and does not induce hepatic enzymes or inhibit metabolism of other antiepileptic drugs. Learn how gabapentin works by binding calcium channel subunits. Full MOA, uses, MCQs, FAQs, and pharmacology inside. In addition, gabapentin does not undergo hepatic metabolism, unlike most other antiepileptic drugs, and is eliminated almost entirely by renal excretion with a clearance that approximates the glomerular filtration rate. Absorption of gabapentin is solely dependent on LAT that are easily saturable, resulting in dose-dependent pharmacokinetics. As the dose of gabapentin increases, the area under the plasma concentration–time curve (AUC) does not increase proportionally. DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. Abstract Gabapentin is approved for the treatment of postherpetic neuralgia (PHN) and epilepsy. The pharmacokinetic (PK) properties of gabapentin, including absorption, distribution, metabolism, and excretion (ADME), were investigated during the development of Neurontin®, an immediate-release (IR) formulation of gabapentin that is orally administered three-times daily. Recently, a Metabolism: In humans, gabapentin undergoes minimal metabolic alteration, largely retaining the original structure. Gabapentin does not induce or inhibit CYP enzymes. Gabapentin is an anticonvulsant drug, which presents an established clinical efficacy in human patients for the management of refractory partial seizures, secondarily generalized tonic-clonic seizures, and for the control of chronic neuropathic pain. Gabapentin was synthesized as a structural analogue of the inhibitory neurotransmitter GABA, with GABA-mimetic effects, able to cross the blood
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |