Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 |  |
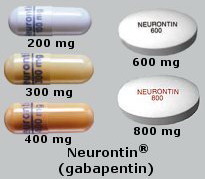
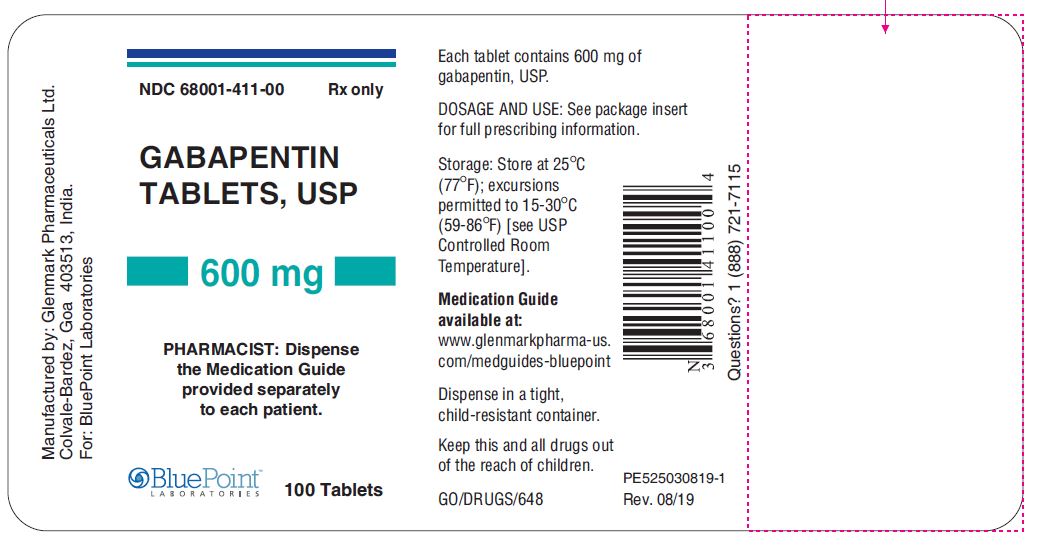
Gabapentin — an anti-seizure drug also approved for nerve pain related to shingles — is now among the most widely prescribed drugs in the country. It's used off-label to treat menopausal hot FDA is warning that serious breathing difficulties may occur in patients using gabapentin or pregabalin who have respiratory risk factors. Gabapentin gained FDA approval in 1993 under the brand name Neurontin as an adjunctive therapy in the treatment of partial onset seizures, and subsequently for the treatment of postherpetic neuralgia in adults in 2002. It became available as a generic in 2004. Gabapentin (Neurontin®, Gralise®) is FDA-approved for use in adults with postherpetic neuralgia (PHN). Neurontin® is also approved as adjunctive therapy for managing partial seizures with or without secondary generalization in patients with epilepsy.1-4 Gabapentin enacarbil (Horizant®) is DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. NEURONTIN® (gabapentin) capsules, for oral use NEURONTIN® (gabapentin) tablets, for oral use NEURONTIN® (gabapentin) oral solution Initial U.S. Approval: 1993 Important Limitation: GRALISE is not interchangeable with other gabapentin products because of differing pharmacokinetic profiles that affect the frequency of administration (See Warnings and Precautions) In December 1993, the US Food and Drug Administration (FDA) granted approval for gabapentin, under the brand name Neurontin, for adjunctive therapy of partial seizures. Subsequently, the FDA approved gabapentin in 2000 for treatment of partial seizures in children aged 3 years or older and in 2002 DESCRIPTION Neurontin® (gabapentin) capsules, Neurontin® (gabapentin) tablets, and Neurontin® (gabapentin) oral solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. Gabapentin and pregabalin are FDA-approved for a variety of uses include fibromyalgia and restless legs syndrome. Gabapentin was first approved in 1993 and pregabalin was When prescribing gabapentin carefully evaluate patients for a history of drug abuse and observe them for signs and symptoms of gabapentin misuse or abuse (e.g., development of tolerance, self-dose escalation, and drug-seeking behavior). Gabapentin is an anticonvulsant (antiseizure) medication approved by the FDA to treat several conditions. Doctors sometimes prescribe gabapentin "off-label" to treat other conditions as well. A 2022 report stated that gabapentin was among the 10 most commonly prescribed medications in the U.S. What is gabapentin and what is it used for? Gabapentin is used to control seizures, to treat nerve FDA approval history for Gralise (gabapentin) used to treat Postherpetic Neuralgia. Supplied by Depomed, Inc. DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. GABAPENTIN (Trade Name: Neurontin®) Introduction: Gabapentin is a prescription medication approved by the United States Food and Drug Administration (FDA) for the treatment of neuropathic pain and epileptic disorders. This drug is currently marketed in capsule, tablet, and oral solution formulations. Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. However, it was later discovered that gabapentin has the potential of an anticonvulsive medication and can be used as an adjunct to more FDA warns about serious breathing problems with seizure and nerve pain medicines gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica CR) When used with CNS depressants or in There are rare postmarketing reports of individuals experiencing withdrawal symptoms shortly after discontinuing higher than recommended doses of gabapentin used to treat illnesses for which the drug is not approved. Note: If you need help accessing information in different file formats, see . Language Assistance Available: | | | | | | | | | | | | | | | Gabapentin was first approved by the U.S. Food and Drug Administration (FDA) for the treatment of seizures in 1993 and was subsequently approved for one pain indication, postherpetic neuralgia.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 |  |