Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
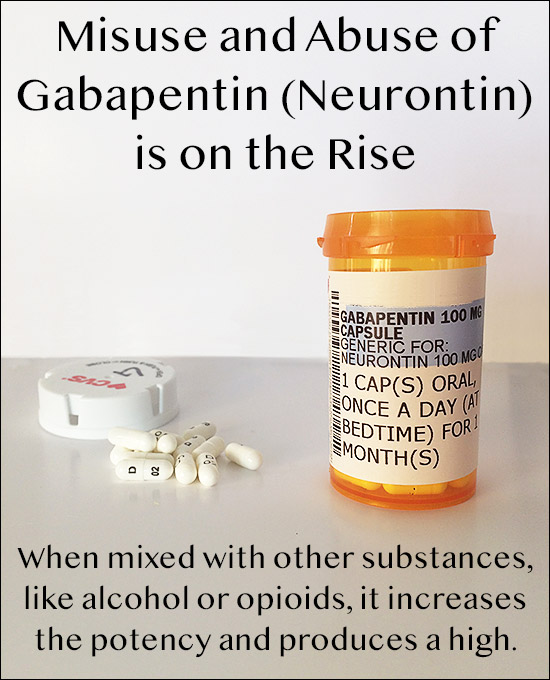
Clinicians who prescribe gabapentinoids off-label for pain should be aware of the limited evidence and should acknowledge to patients that potential benefits are uncertain for most off-label uses. Gabapentinoid drugs—specifically gabapentin (Neurontin) and pregabalin (Lyrica)—are increasingly being prescribed for pain because physicians and patients seek alternatives to opioids in the Off-label gabapentin (Neurontin) got a bad rep when it missed the mark in bipolar disorder, but there may be something worth salvaging in this drug. Here, we weigh its pros and cons for anxiety, substance use disorders, sleep, pain, and hot flashes, and compare it to its underutilized cousin, pregabalin (Lyrica). Gabapentin is widely prescribed off label in medical practice, including psychiatry. The U.S. Food and Drug Administration (FDA) warned of risks associated with gabapentin combined with central nervous system depressant (CNS-D) drugs, which are commonly prescribed in psychiatric treatment. This study examined off-label outpatient gabapentin use for psychiatric indications and concomitant CNS-D Despite limited indications, gabapentin and its cousin, pregabalin (Lyrica), are widely prescribed off-label for various other pain syndromes. Use of gabapentin has increased since 2008; use of pregabalin has remained steady. Between 2017 and 2021, most patients using gabapentinoids reported having musculoskeletal pain and diabetes. Abstract Objective: The objective of the study was to explore the experiences of physicians prescribing gabapentin off label. Methods: We used a case study approach to explore the experiences of physicians prescribing gabapentin for off-label indications. Semi-structured interviews were conducted with 10 physicians (psychiatry, pain and neurology specialists) in the Greater Toronto Area. Data Gabapentin is widely used in the United States for a number of off-label indications, often as an alternative to opioid therapy. Increasing evidence has emerged suggesting that gabapentin may not be as benign as once thought and may be associated In December 1993, the US Food and Drug Administration (FDA) granted approval for gabapentin, under the brand name Neurontin, for adjunctive therapy of partial seizures. Subsequently, the FDA approved gabapentin in 2000 for treatment of partial seizures in children aged 3 years or older and in 2002 The anticonvulsant drug gabapentin is prescribed to treat numerous symptoms and conditions beyond those for which it's approved by the FDA. Here are some common off-label uses and how the evidence stacks up for each. The sustained upward trend in off-label prescribing of gabapentin is concerning, considering the minimal high-quality evidence for many off-label uses. Recent attention to off-label use of gabapentin is largely attributable to a renewed interest in this medication as a chronic pain treatment in the wake of the opioid epidemic. SUMMARY: Gabapentin is approved by the U.S. Food and Drug Administration (FDA) for adjunctive therapy in treatment of partial seizures and postherpetic neuralgia. Various off-label (unapproved) uses have been reported, and the use of gabapentin for off-label purposes has reportedly exceeded use for FDA-approved indications. Pharmaceutical marketing practices and physician dissat-isfaction with The rise in gabapentin prescribing is multifactorial but thought to be due in part to efforts by the pharmaceutical industry to promote the use of the medication for off-label uses. (In 2004, the manufacturer of Neurontin, Pfizer, pleaded guilty to multiple counts of illegally promoting the off-label use of gabapentin, resulting in nearly $430 million in fines.) Additionally, gabapentinoids In today’s video, we explore the off-label uses of Gabapentin, also known as Neurontin. While Gabapentin is FDA-approved for partial seizures and postherpetic neuralgia, its off-label uses are more extensive, especially in psychiatry. Gabapentin (Neurontin) and pregabalin (Lyrica) are anticonvulsants and nerve pain medicines which have structural similarities to the inhibitory neurotransmitter GABA. Gabapentin was developed in 1993 and has indications for shingles (‘postherpetic neuralgia’) and partial-onset seizures. It has had a growing popularity in off-label uses for fibromyalgia, pain from a variety of causes Gabapentin is widely used in the United States for a number of off-label indications, often as an alternative to opioid therapy. Increasing evidence has emerged suggesting that gabapentin may not be as benign as once thought and may be associated with substance abuse in concert with opioids. With co Gabapentin is an anticonvulsant (antiseizure) medication approved by the FDA to treat several conditions. Doctors sometimes prescribe gabapentin "off-label" to treat other conditions as well. A 2022 report stated that gabapentin was among the 10 most commonly prescribed medications in the U.S. What is gabapentin and what is it used for? Gabapentin is used to control seizures, to treat nerve Off-Label Usages of Gabapentin - Gabapentin - GabapentinGabapentin is frequently prescribed off-label for a variety of conditions outside its primary approvals for epilepsy and nerve pain. Gabapentin (Neurontin) is not a medication that would make the FDA proud. Less than 1% of its outpatient use is for an FDA indication, and a good portion of the off-label use takes place in psychiatry. These Off-label: It is estimated that approximately 9/10 prescriptions for Gabapentin are “off-label” or for conditions that the drug isn’t approved to treat. Off-label prescriptions have a reduce chance of actually working for the treatment of anxiety. The drug Neurontin is used as an example of why it is permissible for physicians to engage in off-label prescribing, but off-label marketing by pharmaceutical companies is prohibited by the FDA.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |