Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 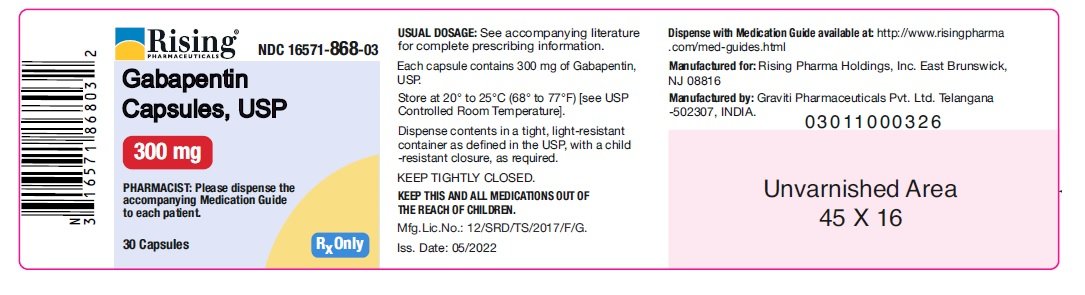 |
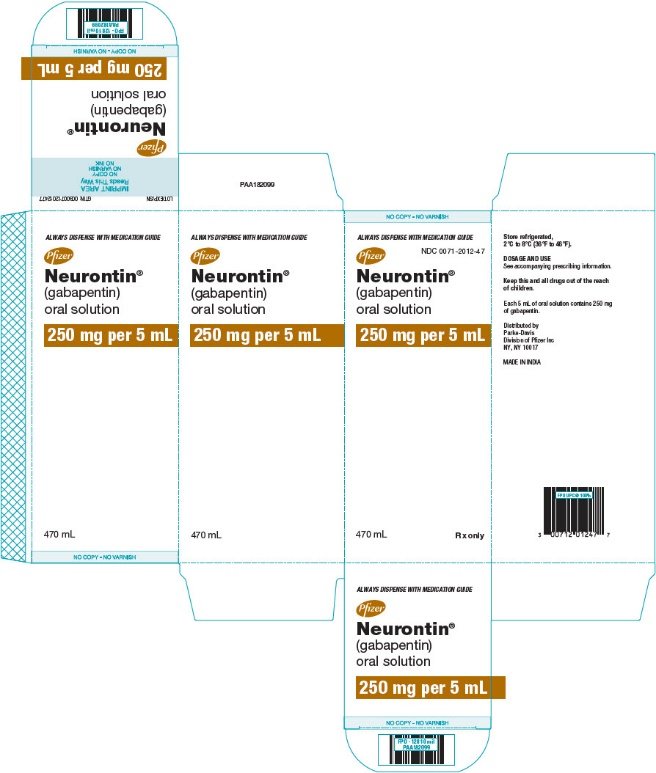
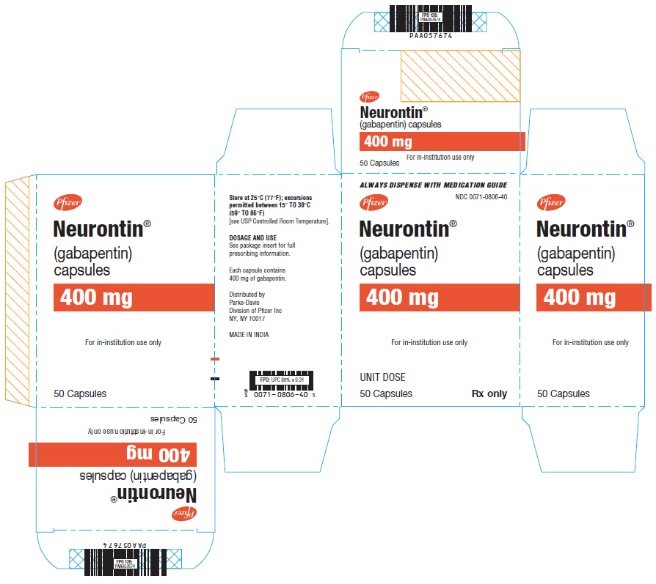
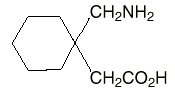
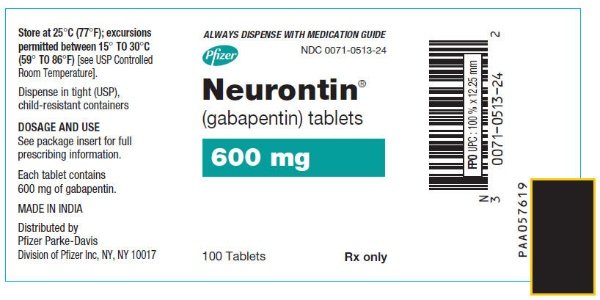
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GABAPENTIN safely and effectively. See full prescribing information for GABAPENTIN. Gabapentin package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. Who should not take gabapentin? Do not take gabapentin if you are allergic to gabapentin or any of the other ingredients in gabapentin. See the end of this Medication Guide for a complete list of ingredients in gabapentin. What should I tell my healthcare provider before taking gabapentin? Before taking gabapentin, tell your healthcare provider DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. These highlights do not include all the information needed to use GABAPENTIN CAPSULES safely and effectively. See full prescribing information for GABAPENTIN CAPSULES. GABAPENTIN capsules, for oral use Initial U.S. Approval: 1993 DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. DESCRIPTION Each capsule contains: 300 mg of gabapentin, USP. DOSAGE & ADMINISTRATION Dosage and Use: See package insert - for full prescribing information STORAGE AND HANDLING Store at 20 to 25 C (68 to 77 F); excursions - permitted to 15 to 30 C (59 to 86 F)) [See - USP Controlled Room Temperature]. Dispense in tight (USP), child-resistant 2.1 Dosage for Postherpetic Neuralgia In adults with postherpetic neuralgia, NEURONTIN may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). The dose can subsequently be titrated up as needed for pain relief to a dose of 1800 mg/day (600 mg three times a day). In clinical studies, efficacy was Quality Care Products, LLC: Gabapentin is indicated for: Management of postherpetic neuralgia in adults. Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in Neurontin package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. This product information is intended only for residents of the United States. In order to properly inform the public, we have developed the following statement to be placed at the top of certain product pages: The products on this page have been transferred to VIATRIS SPECIALTY LLC. The U.S. Physician Prescribing Information and Patient Information on this page may no longer be current. Please Viatris Specialty LLC: NEURONTIN is indicated for: Management of postherpetic neuralgia in adults. Adjunctive therapy in the treatment of partial onset seizures, with and without secondary 2.1 Dosage for Postherpetic Neuralgia In adults with postherpetic neuralgia, NEURONTIN may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). The dose can subsequently be titrated up as needed for pain relief to a dose of 1800 mg/day (600 mg three times a day). In clinical studies, efficacy was In adults with postherpetic neuralgia, NEURONTIN may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). The dose can subsequently be titrated up as needed for pain relief to a dose of 1800 mg/day (600 mg three times a day). The active ingredient in NEURONTIN capsules, tablets, and oral solution is gabapentin, which has the chemical name 1- (aminomethyl)cyclohexaneacetic acid. The molecular formula of gabapentin is Becoming dependent on Neurontin (‘drug dependence’) After stopping a short or long-term treatment with Neurontin, you need to know that you may experience certain side effects, so-called withdrawal effects (see “If you stop taking Neurontin”). The recommended maintenance dose of NEURONTIN in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. NEURONTIN may be administered as the oral solution, capsule, or tablet, or using combinations of these formulations. Dosages up to 50 mg/kg/day have been well tolerated in a long-term clinical study. Gabarone package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. These highlights do not include all the information needed to use NEURONTIN safely and effectively. See full prescribing information for
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |