Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
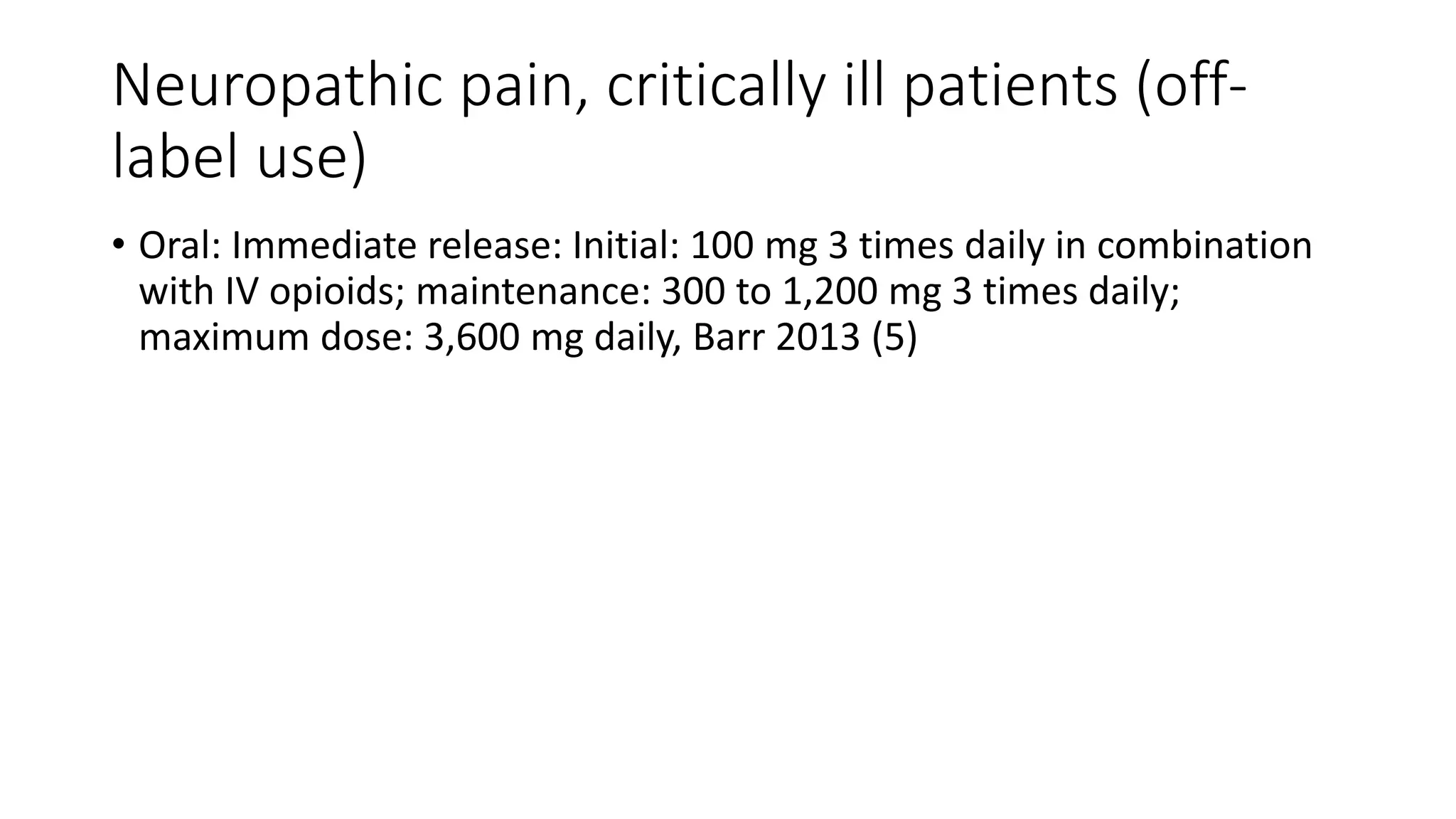
+Indications:+SPS%2C+CPS%2C+w/wo+2ary+GTCS.+Add-on+therapy.+Side+Effects:+Usual+AED+AEs..jpg) | 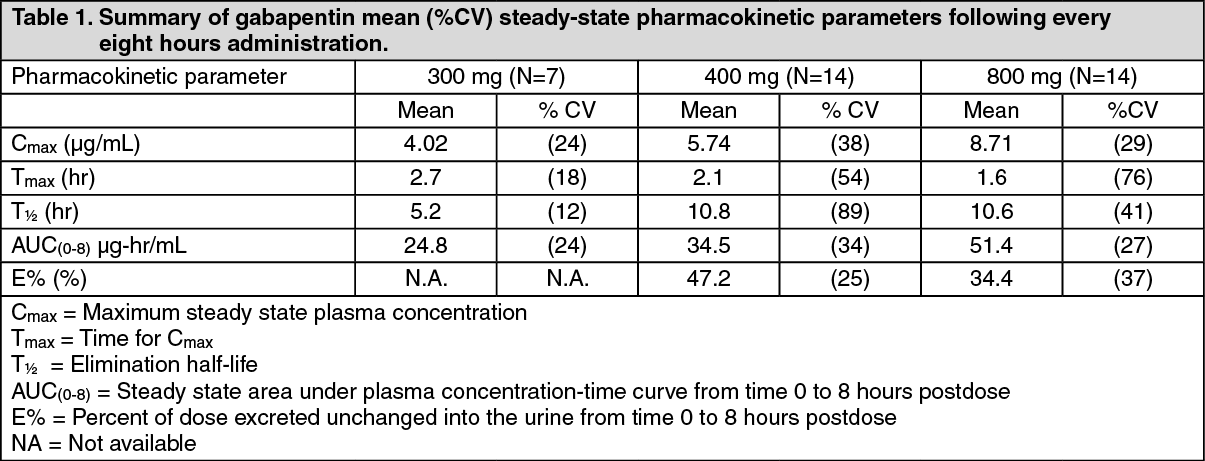 |
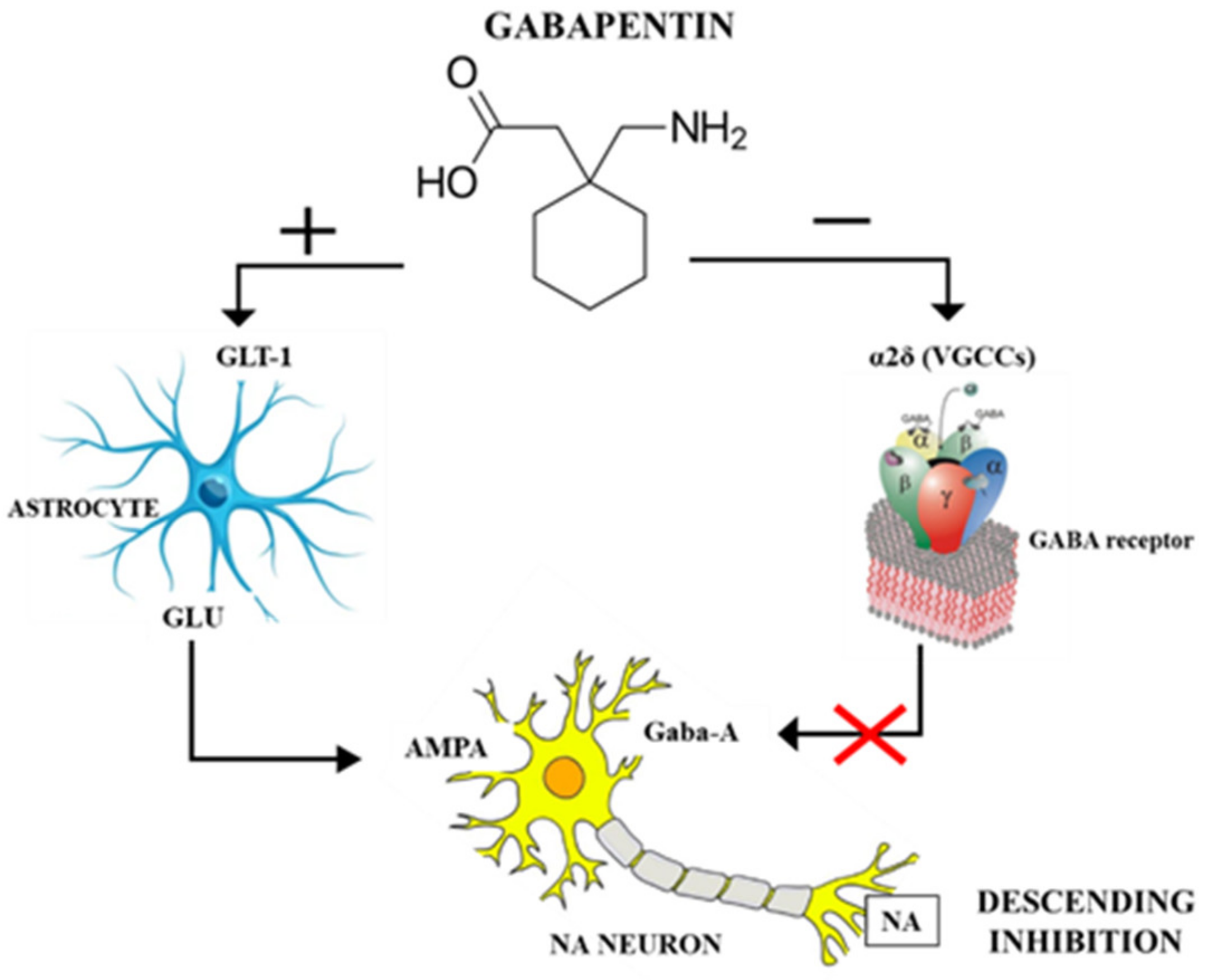
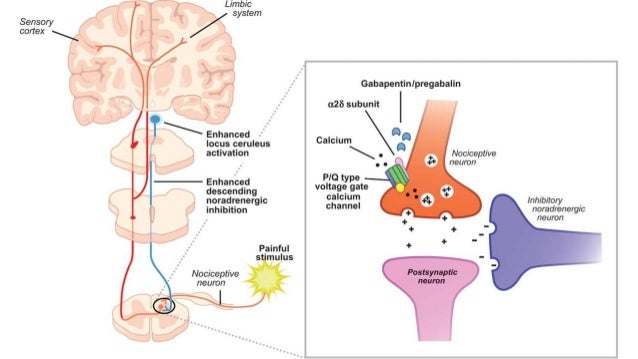
Pregabalin and gabapentin share a similar mechanism of action, inhibiting calcium influx and subsequent release of excitatory neurotransmitters; however, the compounds differ in their pharmacokinetic and pharmacodynamic characteristics. Gabapentin is absorbed slowly after oral administration, with m 12. Bockbrader HN, Wesche D, Miller R, et al. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinet 2010; 49: 661–669. Includes Gabapentin indications, dosage/administration, pharmacology, mechanism/onset/duration of action, half-life, dosage forms, interactions, warnings, adverse reactions, off-label uses and more. Gabapentin Pharmacokinetics Absorption Bioavailability Conventional (immediate-release) gabapentin: Following oral administration, absorbed principally in the proximal small intestine via a saturable L-amino acid transport system; bioavailability is not dose proportional and decreases as dose increases. Bioavailability is 60 to 27% for doses ranging from 900 mg to 4.8 g daily. Gabapentin Gabapentin, sold under the brand name Neurontin among others, is an anticonvulsant medication primarily used to treat neuropathic pain and also for partial seizures [10][7] of epilepsy. DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. 12. Bockbrader HN, Wesche D, Miller R, et al. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinet 2010; 49: 661–669. Keywords: Gabapentin, pregabalin, pain management, adverse effects, pharmacology Introduction The gabapentinoid drugs gabapentin and pregabalin are antiepileptic drugs that are considered as first-line treatments for the management of neuropathic pain. 1 Pregabalin is also approved for generalised anxiety disorders in the United Kingdom. Pharmacokinetics Not appreciably metabolized in humans Eliminated from the systemic circulation by renal excretion Elimination half-life ≅ 5 to 7 hours In elderly patients and those with impaired renal function, plasma clearance is reduced Mean half-life increased from 6.5 h (CrCl >60 mL/min) to 52 h (CrCL <30 mL/min) Gabapentin can be removed from plasma by hemodialysis Bioavailability is Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. However, it was later discovered that gabapentin has the potential of an anticonvulsive medication and can be used as an adjunct to more The gabapentinoids are often recommended as first-line treatments for the management of neuropathic pain. The differing pharmacodynamic and pharmacokinetic profiles can have implications for clinical practice. This article has summarised these key differences. In addition to their use in managing ne Gabapentin (“Neurontin”) has been shown in extensive preclinical and clinical studies to be an effective anticonvulsant drug, which appears to have novel mechanisms of action. Although designed as a GABA analogue it is clearly not GABAmimetic, although Pharmacokinetic properties are important to consider in evaluating the usefulness of new antiepileptic drugs (AEDs). Gabapentin is a new, water-soluble, antiepileptic agent with properties of an amino acid. This drug is rapidly absorbed and exhibits dose-dependent bioavailability as a result of a sa Gabapentin is a new antiepileptic drug (AED) with an attractive pharmacokinetic profile. It is absorbed by an active and saturable transport system, and has a high volume of distribution. Gabapentin is not bound to plasma proteins, does not induce hepatic enzymes and is not metabolized. At steady st In a single (400 mg) and multiple dose (400 mg three times a day) study of NEURONTIN in epileptic patients (N=8) maintained on phenytoin monotherapy for at least 2 months, gabapentin had no effect on the steady-state trough plasma concentrations of phenytoin and phenytoin had no effect on gabapentin pharmacokinetics. Gabapentin is an anticonvulsant medication used in the management of peripheral neuropathic pains, postherpetic neuralgia, and partial-onset seizures. Background/Objectives: Gabapentin has variable pharmacokinetics (PK), which contributes to difficulty in dosing and increased risk of adverse events. The objective of this study was to leverage gabapentin concentrations from therapeutic drug monitoring (TDM) to develop a population PK (popPK) model and characterize significant covariates that impact gabapentin PK. Methods: Data were The gabapentinoids are often recommended as first-line treatments for the management of neuropathic pain. The differing pharmacodynamic and pharmacokinetic profiles can have implications for Introduction The gabapentinoid drugs gabapentin and pregabalin are antiepileptic drugs that are considered as first-line treatments for the management of neuropathic pain.1 Pregabalin is also approved for generalised anxiety dis-orders in the United Kingdom. The mechanisms of action are still unclear despite their widespread use. The gabapentinoids share similar mechanisms of action but differ Epidemiologic data indicates that the occurrence of epilepsy increase significantly after age 60. 36 Features complicating the treatment of seizures in older age groups consist of simultaneous medical diseases, polytherapy, alteration in pharmacokinetics (pK), and changed CNS pharmacodynamics. 37 Gabapentin seemed to have promising
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
+Indications:+SPS%2C+CPS%2C+w/wo+2ary+GTCS.+Add-on+therapy.+Side+Effects:+Usual+AED+AEs..jpg) |  |