Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
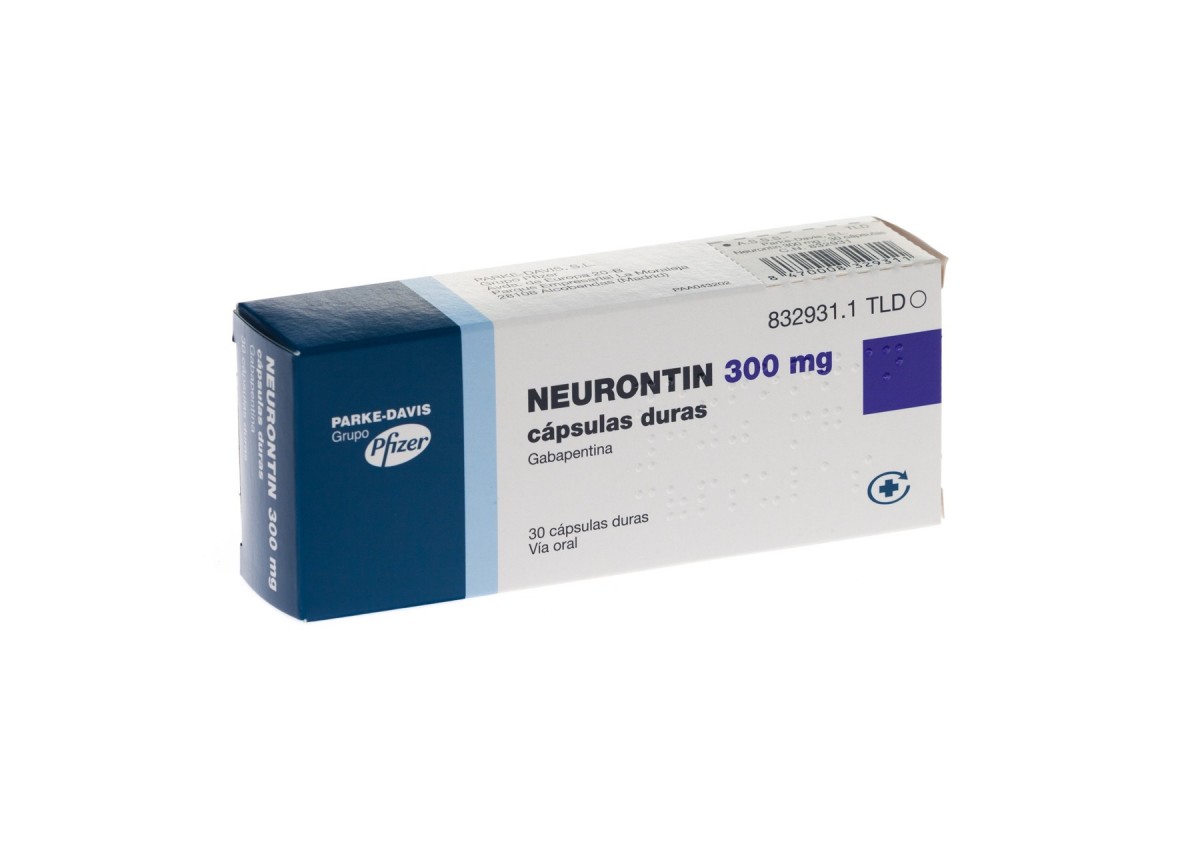 |  |
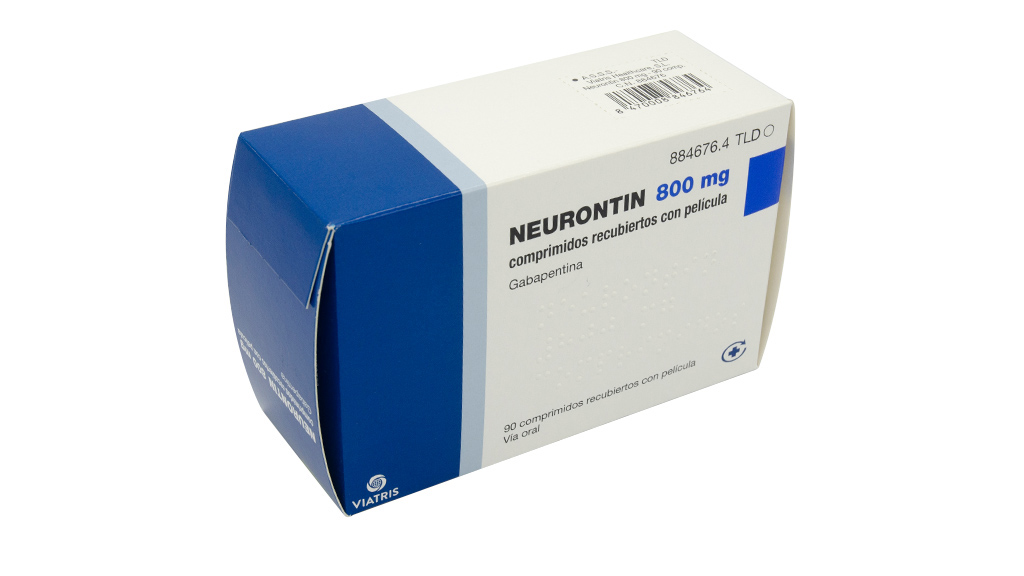
Introduction: Gabapentin is a prescription medication approved by the United States Food and Drug Administration (FDA) for the treatment of neuropathic pain and epileptic disorders. This drug is currently marketed in capsule, tablet, and oral solution formulations. In recent years, however, gabapentin has been increasingly encountered by law enforcement, documented in national crime lab RX DRUG SCHEDULING & MONITORING Prescription Drug Monitoring Programs (PDMPs) are electronic databases that collect information on the dispensing and prescribing of drugs within jurisdictions. PDMPs aim to assist patients in their quality of care by allowing prescribers and dispensers access to the patient’s controlled substance prescription medication history. This access to individual II: N Adzenys ER/XR-ODT, Dyanavel XR, Evekeo, Evekeo ODT Anileridine 9020 II Y Leritine Benzhydrocodone combination products 9193 II Y Apadaz Bezitramide 9800 II Y Burgodin Carfen Individuals at the highest risk for abusing gabapentin include those with opioid abuse, mental illness, or previous history of prescription drug abuse. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. Regional Variation Gabapentin’s regulatory status varies by state. Some states classify it as a Schedule V controlled substance due to concerns about misuse and its involvement in the opioid crisis. Others do not schedule it but require mandatory reporting to state prescription drug monitoring programs (PDMPs) to track prescribing and dispensing. This annual publication of the Texas Schedules of Controlled Substances was signed by Jennifer A. Shuford, M.D., M.P.H., Commissioner of Health, and will take effect 21 days following publication of this notice in the Texas Register. Drug Schedules Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules depending upon the drug’s acceptable medical use and the drug’s abuse or dependency potential. The abuse rate is a determinate factor in the scheduling of the drug; for example, Schedule I drugs have a high potential for abuse and the potential to create 9.1 Controlled Substance - Gabapentin is not a scheduled drug. 9.2 Abuse - Abuse is the intentional, non-therapeutic use of a drug, even once, for its desirable psychological or physiological effects Presently, seven states have classified gabapentin as a Schedule V controlled substance, and 12 others, New Jersey included, require that gabapentin prescriptions be reported in the PDMP system. Every time a prescription for gabapentin is filled out, it will automatically be added to the database. Gabapentin is a prescription medication approved by the FDA for the treatment of neuropathic pain (postherpetic neuralgia) and seizure disorders. Why is gabapentin controlled in some states? Gabapentin is structurally and pharmacologically related to pregabalin (Lyrica, Lyrica CR), which is a Schedule V drug and controlled federally in all states. Individuals at the highest risk for abusing gabapentin include those with opioid abuse, mental illness, or previous history of prescription drug abuse. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. Neurontin package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. Between August 2016 and July 2018, three states classified gabapentin as a Schedule V drug and nine states implemented prescription drug monitoring program (PDMP) regulation for gabapentin. It is highly unusual for states to take drug regulation We would like to show you a description here but the site won’t allow us. The list of controlled substances posted on the DPH website is for informational purposes only. The posted list includes all substances scheduled in accordance with the provisions of SC Code Section 44-53-160, designated by DPH and transmitted to the General Assembly since the last SC Code update in 2012, as well as the list of scheduled substances currently in the SC Code for Schedules I-V Gabapentin isn't a narcotic, but it is a controlled substance in some states. Here's what you should know before using it. Gabapentin – or Neurontin – is a medication commonly used to treat nerve pain and seizures. However, the drug can have potentially harmful effects when combined with other opioids. Michigan joins a growing number of states that have scheduled Gabapentin as a controlled substance. Individuals at the highest risk for abusing gabapentin include those with opioid abuse, mental illness, or previous history of prescription drug abuse. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. Schedule V drugs have a lower potential for abuse than all other controlled substances. This means gabapentin has a lower risk of abuse compared to Oxycontin (oxycodone), which is a schedule II opioid medication. Gabapentin is a controlled substance in states like Michigan and Kentucky, while others have mandated reporting rules. Learn about its risk for abuse here.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |