Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 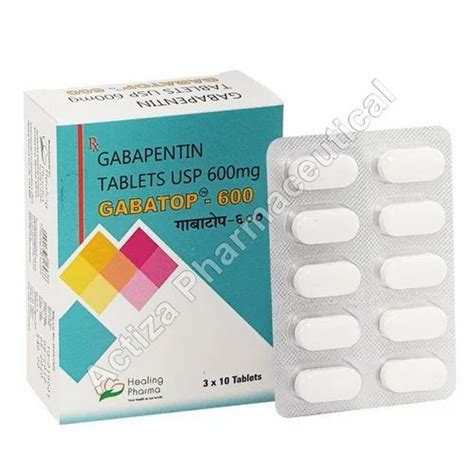 |
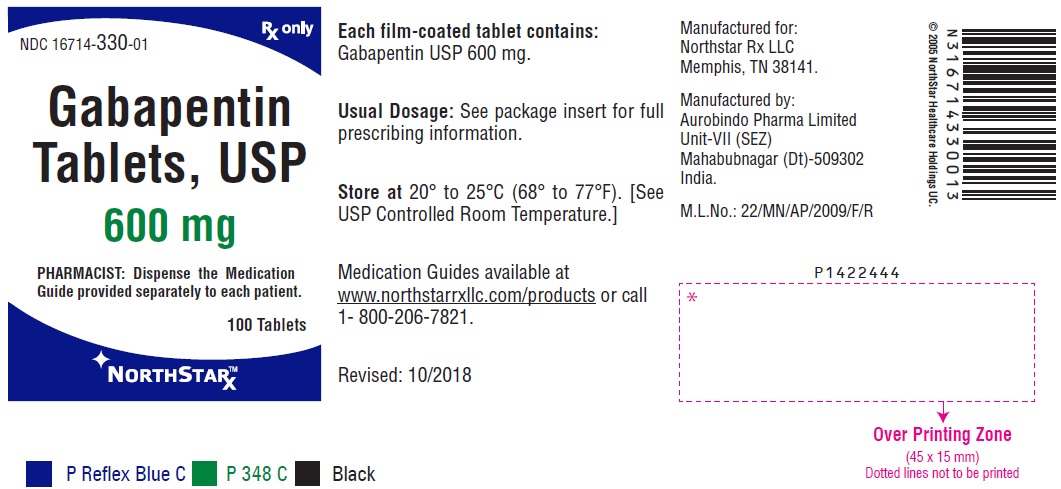
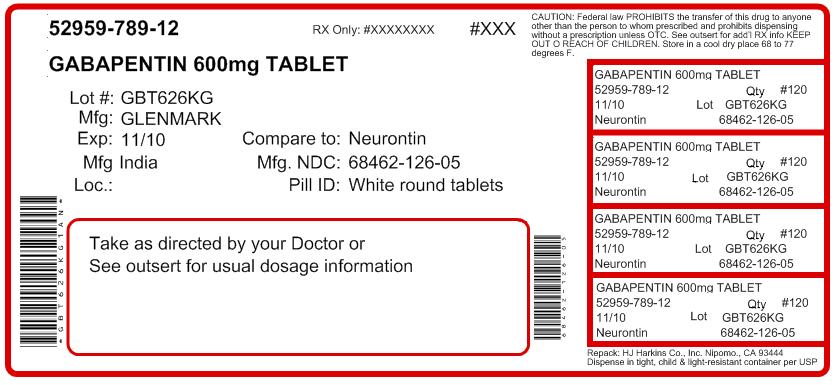
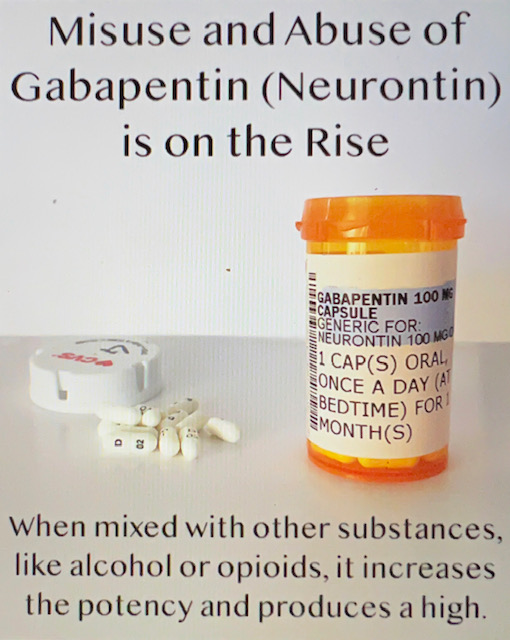
A US-based subsidiary of Sun Pharma is recalling around 13,700 bottles of Gabapentin capsules, a medication used to treat and prevent seizures in people with epilepsy. Drug Recall Enforcement Report Class II voluntary initiated by Granules Pharmaceuticals Inc., initiated on 07-31-2024 for the product Gabapentin Tablets, USP, A recall has been issued for almost 4000 cartons of gabapentin tablets distributed throughout the United States due to contamination. Read more on Pharmacy Learning Network. The Harvard Drug Group, LLC d/b/a Major® Pharmaceuticals and Rugby® Laboratories is initiating a recall of Gabapentin Capsules, USP, 100 mg. This recall has been initiated due to receiving reports of inadequately sealed blister packaging. Nearly 40 generic medications—most prescription, but some sold over-the-counter at Amazon and Walmart—have been recalled due to manufacturing quality concerns, the FDA said. See the full list The FDA has reported out that, Sun Pharmaceutical Industries, Inc. initiated a recall for 13,728 bottles of gabapentin, including 12,876 bottles containing 300-milligram dosage and 852 bottles containing 400 milligrams per dose. The Board of Pharmacy has received notice of the following product recall. The Board strongly encourages pharmacies to immediately review their quality assurance and recall policies and procedures to determine if any corrective action is required. The Harvard Drug Group, LLC d/b/a Major Pharmaceuticals and Rugby Laboratories has initiated a recall for Gabapentin Tablets, 600 mg. This recall Drug Recall Enforcement Report Class III voluntary initiated by Sciegen Pharmaceuticals Inc, originally initiated on 02-17-2023 for the product Gabapentin Tablets, USP 600 mg, 500 tablets per bottle, RX Only, Manufactured by ScieGen Pharmaceuticals Inc., Hauppauge, NY 11788, NDC: 50228-177-05. The product was recalled due to presence of foreign tablets/capsules: pharmacist reported presence of The FDA has reported out that, Sun Pharmaceutical Industries, Inc. initiated a recall for 13,728 bottles of gabapentin, including 12,876 bottles containing 300-milligram dosage and 852 bottles Four gabapentinoids have new warnings about the risk of suicidal thoughts and the risk of withdrawal symptoms. The FDA sent letters to the sponsors of these products with instructions to add new safety information to drug labels: Neurontin (gabapentin), Gralise (gabapentin), Horizant (gabapentin enacarbil), Lyrica and Lyrica CR (pregabalin). The U.S. Food and Drug Administration (FDA) has reported that Aurobindo Pharma USA is voluntarily recalling Gabapentin 300 mg capsules from lot GESB14011-A in 100-count bottles. Description: Gabapentin Tablets, USP 600 mg, packaged in Cartons of 100 tablets (10 tablets per blister pack x 10), Rx Only, Distributed by: Aurobindo Pharma USA, Inc. East Windsor, NJ 08520 Distributed by: Major Pharmaceuticals 17177 N Laurel Park Dr., Suite 233 Livonia, MI 48152 USA, NDC 0904-6823-61 The most recent Recall Enforcement Report that covers this product was initiated on July 31st, 2024 and classified as a Class II recall due to presence of foreign tablets; 3 fused tablets of metformin er 500 mg were found in bottle of gabapentin tablets This recall is currently completed, and the associated recall number is recall number is D-0634-2024. It pertains to Gabapentin identified by FDA warns about serious breathing problems with seizure and nerve pain medicines gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica CR) FDA Drug Safety Podcast Get an alert when a recall is issued. Do not use • if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. This recall is due to a potential mix-up of Metformin ER Tablets in a bottle of Gabapentin Tablets, USP 600mg. The ongoing investigation revealed that the issue is limited to the above lot and no other lots were impacted. The recall affects 13 728 bottles—12 876 bottles of 300 mg capsules and 852 bottles of 400 mg capsules—due to concerns of cross contamination during manufacturing. The impacted lots, with expiration dates ranging from March to August 2025, were distributed nationwide across the United States. Description: Gabapentin Tablets 600mg, 500-count bottles, Rx Only, Manufactured by: Glenmark Pharmaceuticals Limited, Pithampur, Madhya Pradesh 454775, India, Manufactured for: Glenmark Pharmaceuticals Inc. USA, Mahwah, NJ 07430. NDC# 68462-126-05. The FDA will determine if a firm's removal or correction actions of a marked product meet the requirements to be considered a "recall". The FDA will also determine the public hazard assessment and a recall classification will be published in the Enforcement Report. The recall stems from violations of the FDA’s Current Good Manufacturing Practice (CGMP) standards—rules that ensure medications are made safely and consistently. The recall is classified as Class II, which the FDA defines as a product that could cause temporary or medically reversible health effects or where there is a low risk of serious
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |