Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 | |
 |
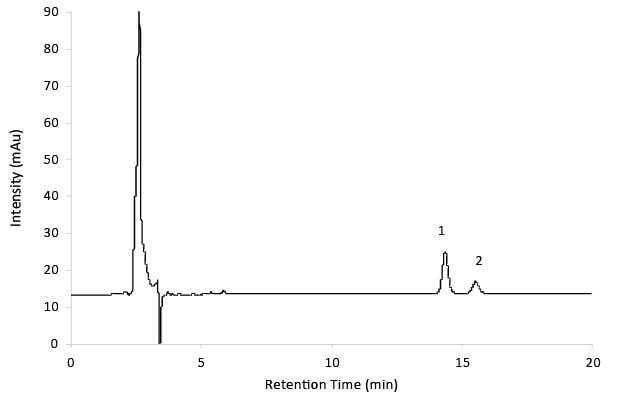
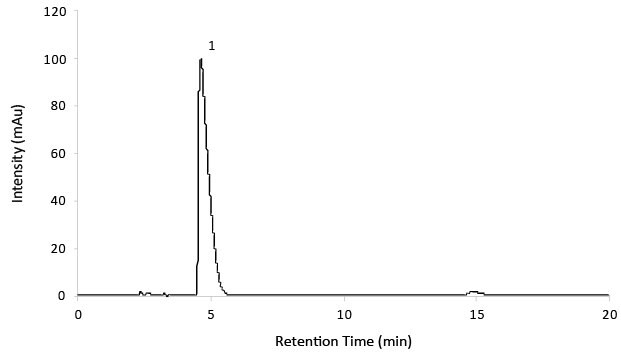
Product Details Teva-Gabapentin ODB Formulary Therapeutic Classification 28:12:00 Anticonvulsants Therapeutic Note NO ATC Code N02BF01 Interchangeable Products LU Clinical Criteria NO EAP Criteria NO Product Monograph View Monograph The Patient Information Leaflet for products containing Gabapentin from Teva can be downloaded below, along with other information such as Frequently Asked Questions where applicable. 1 NAME OF THE MEDICINAL PRODUCT Gabapentin Teva 50 mg/ml oral solution. Teva-Gabapentin: Gabapentin belongs to the class of medications called anti-epileptics. It is used in combination with other seizure control medications to manage and prevent seizures associated with epilepsy. As Canada's trusted pharmacy, Rexall provides detailed drug factsheets for Teva-Gabapentin with common uses, dosage instructions, side effects & drug interactions. In vitro studies with radiolabeled gabapentin have revealed a gabapentin binding site in areas of rat brain including neocortex and hippocampus. A high-affinity binding protein in animal brain Consumers or patients Product monographs and consumer information for Teva Canada products can be found in the Health Canada Drug Product Database. Consult your healthcare practitioner if you have any questions about a medication you are taking. A double-blind, randomized, two-way crossover, single-dose (1 x 400 mg) bioequivalence study of GABAPENTIN 400 mg capsules (Teva Canada Limited) and NEURONTIN® 400 mg capsules (Upjohn EESV) was conducted in 38 healthy adult male subjects under fasting conditions. TEVA-GABAPENTIN (gabapentin) is indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. This leaflet is part III of a three-part “Product Monograph" published when TEVA-GABAPENTIN was approved for sale in Canada and is designed specifically for Consumers. Product Details Teva-Gabapentin ODB Formulary Therapeutic Classification 28:12:00 Anticonvulsants Therapeutic Note NO ATC Code N02BF01 Interchangeable Products LU Clinical Criteria NO EAP Criteria NO Product Monograph View Monograph Information on drug and health products authorized by Health Canada. Information on drug and health products authorized by Health Canada. The product monograph is developed by a drug sponsor according to guidelines published by Health Canada that provide direction on the content and format. The veterinary labelling is developed by the drug sponsor according to the Food and Drug Regulations. While Health Canada reviews the product monograph or the veterinary labelling as part of the drug review process, it remains the Adults TEVA-GABAPENTIN (gabapentin) is indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. Save money on your Neurontin® Capsules prescription by switching to Teva's FDA-approved generic version, Gabapentin Capsules, USP Click on a resource to visit a page with more information. You may be taken away from this page to a different Government of Canada website. Information on drug and health products authorized by Health Canada. In adults with postherpetic neuralgia, NEURONTIN may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a SUMMARY PRODUCT INFORMATION INDICATIONS AND CLINICAL USE Adults GABAPENTIN is indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. MYLAN-GABAPENTIN (gabapentin) may be used in combination with other commonly used antiepileptic drugs without concern for alteration of the blood concentrations of gabapentin or other antiepileptic drugs.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 | |
 |