Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
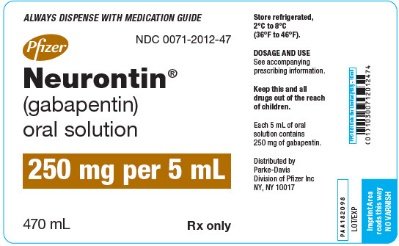
Complete details for NDC 42192-0608-05 Gabapentin 250 mg/5mL including product information, packaging information, pricing, prescribing information and package photos. GPI: 72600030002020 The NDC Packaged Code 42192-608-16 is assigned to a package of 470 ml in 1 bottle of Gabapentin, a human prescription drug labeled by Acella Pharmaceuticals, Llc. Order Gabapentin 250 mg / 5 mL Solution 470 mL by Acella Pharmaceuticals 42192060816 DESCRIPTION Gabapentin Oral Solution is supplied as oral solution containing 250 mg/5 mL of gabapentin. The inactive ingredients are anise flavor natural and artificial, cooling agent flavor artificial, glycerin, purified water, strawberry flavor artificial and xylitol. Gabapentin is described as 1- (aminomethyl) cyclohexaneacetic acid with a molecular formula of C 9 H 17 NO 2 and a molecular The active ingredient in Gabapentin Oral solution is gabapentin, which has the chemical name 1-(aminomethyl)cyclohexaneacetic acid. The molecular formula of gabapentin is C9H17NO2 and the molecular weight is 171.24. For more information about Gabapentin Oral Solution, medical inquiries, or to report side effects regarding Gabapentin Oral Solution, please call 1-800-541-4802. Page 2: Acella Pharmaceuticals, LLC: Gabapentin Oral Solution is indicated for: Management of postherpetic neuralgia in adults. Adjunctive therapy in the treatment of partial onset seizures, with and without secondary GABAPENTIN- gabapentin solution Acella Pharmaceuticals, LLC ---------- HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GABAPENTIN ORAL SOLUTION safely and effectively. See full prescribing information for GABAPENTIN ORAL Page 5: Quality Care Products, LLC: Gabapentin is indicated for: Management of postherpetic neuralgia in adults. Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. The NDC code 42192-608 is assigned by the FDA to the product Gabapentin which is a human prescription drug product labeled by Acella Pharmaceuticals, Llc. The product's dosage form is solution and is administered via oral form. The product is distributed in 5 packages with assigned NDC codes 42192-608-05 5 ml in 1 cup, unit-dose , 42192-608-06 6 ml in 1 cup, unit-dose , 42192-608-16 470 ml in HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GABAPENTIN safely and effectively. See full prescribing information for GABAPENTIN. Page 7: Quality Care Products, LLC: Gabapentin is indicated for: Management of postherpetic neuralgia in adults. Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in Acella Pharmaceuticals, LLC: Gabapentin Oral Solution is indicated for: Management of postherpetic neuralgia in adults. Adjunctive therapy in the treatment of partial onset seizures, with and without secondary The active ingredient in Gabapentin Oral Solution is gabapentin,which has the chemical name 1- (aminomethyl)cyclohexaneacetic acid. The molecular formula of gabapentin is C - 9H Gabapentin oral solution 250 mg per 5 mL, is a clear pale yellow to yellow colored solution and is supplied as follows: Bottle containing 473 mL NDC 65162-698-90 - 50 x 5 mL unit Acella Pharmaceuticals, LLC: Gabapentin Oral Solution is indicated for: Management of postherpetic neuralgia in adults. Adjunctive therapy in the treatment of partial onset seizures, with and without secondary Gabapentin Oral Solution View PI View Medication Guide Hyoscyamine Oral Tablets View PI Who should not take Gabapentin Oral Solution? n Oral Solution. See the end of this Medication Guide for a complete list of ingredients in Gabapent What should I tell my healthcare provider before taking gabapentin? Before taking gabapentin, tell your healthcare provider if you: Quality Care Products, LLC: Gabapentin is indicated for: Management of postherpetic neuralgia in adults. Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |