Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 |  |
 |  |
 | 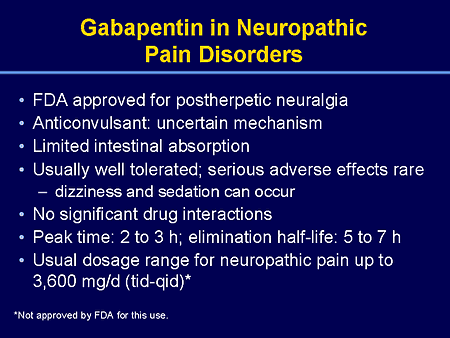 |
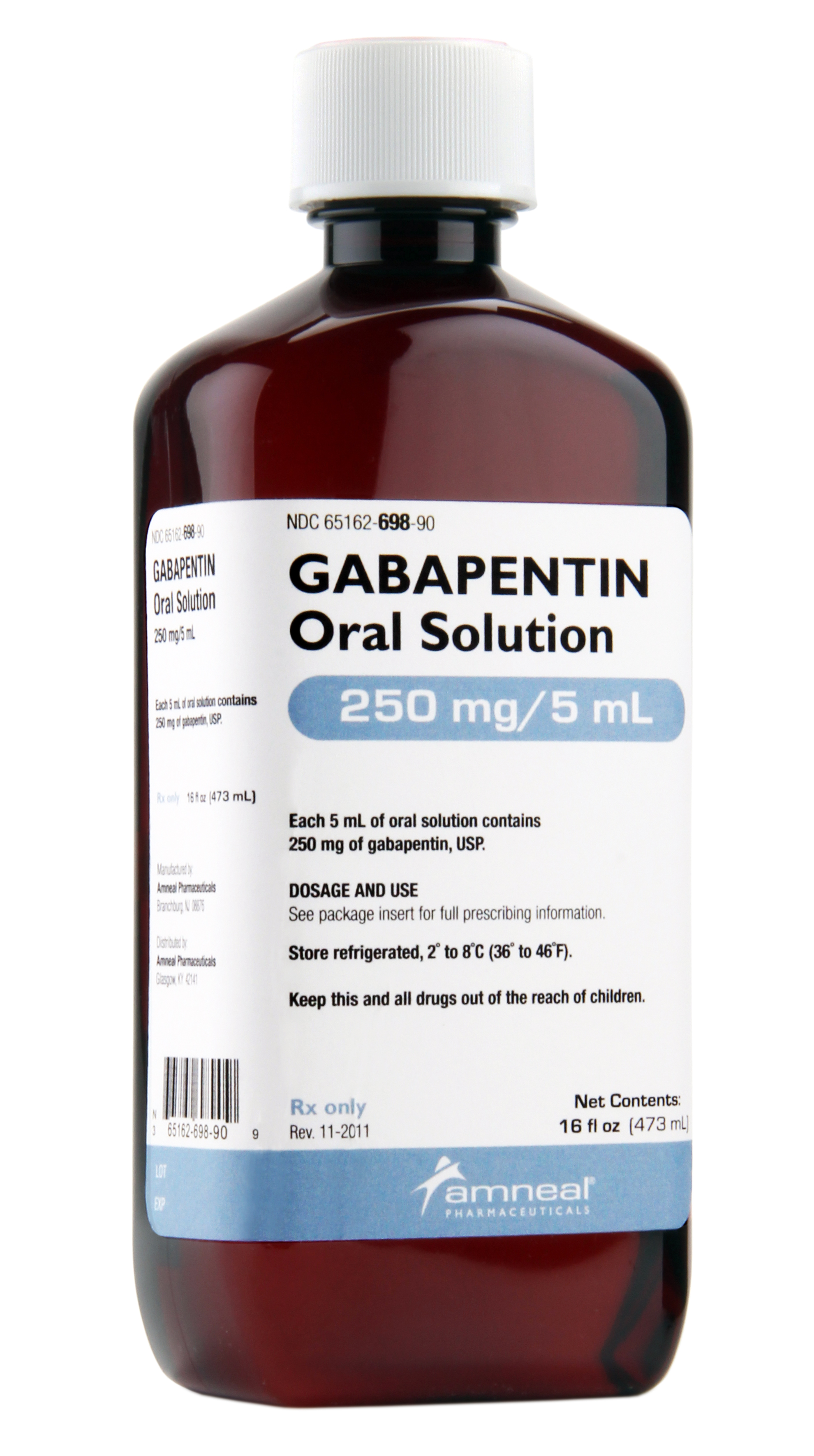
Find patient medical information for Gabapentin (Gralise, Neurontin) on WebMD including its uses, side effects and safety, interactions, pictures, warnings, and user ratings Gabapentin and pregabalin are FDA-approved for a variety of uses include fibromyalgia and restless legs syndrome. Gabapentin was first approved in 1993 and pregabalin was Gabapentin is a prescription drug used to treat seizure disorders and nerve damage from shingles. Off label uses (non-FDA approved) include fibromyalgia, headaches, and hot flashes. Common side effects are fatigue, nausea, hostility, dizziness, and tremors. Gabapentin is not an opioid narcotic, but it does have signs and symptoms associated with drug misuse, addiction, and withdrawal symptoms Gabapentin is widely used in the United States for a number of off-label indications, often as an alternative to opioid therapy. Increasing evidence has emerged suggesting that gabapentin may not be as benign as once thought and may be associated What are the approved indications for gabapentin and pregabalin? What is the evidence for and against their use in various off-label indications, and how can doctors help limit gabapentinoid-related harms? Gabapentin is an anti-epileptic drug, also called an anticonvulsant. It is used to treat some types of seizures and nerve pain caused by shingles. Gabapentin is prescribed to manage conditions affecting the nervous system, helping to alleviate chronic pain, control seizures, and improve neurological discomfort. Gabapentin alters nerve signaling in the brain to reduce pain and seizures. Beyond its approved uses, Gabapentin is increasingly prescribed off-label for conditions like anxiety, migraines, and fibromyalgia. According to a report Here's who gabapentin was originally approved for, what it's used for today and why it's becoming a drug of increasing concern for abuse and misuse. Gabapentin (Neuraptine, Gralise, and Gralise 30-Day Starter Pack) is an anti-seizure (anticonvulsant) medication used to treat seizure disorders and postherpetic neuralgia (the pain that follows an episode of shingles). Off-label uses (uses not approved by the Food and Drug Administration, or FDA) for gabapentin include alcohol withdrawal, cocaine withdrawal, anxiety, hiccups, restless leg Gabapentin is an anticonvulsant medication prescribed for a variety of conditions. Learn about its uses, side effects, and what you should know if you've been prescribed this medication. Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. However, it was later discovered that gabapentin has the potential of an anticonvulsive medication and can be used as an adjunct to more Gabapentin is approved to prevent and control partial seizures, relieve postherpetic neuralgia after shingles and moderate-to-severe restless legs syndrome. Learn what side effects to watch for, drugs to avoid while taking gabapentin, how to take gabapentin and other important questions and answers. Gabapentin is available in both branded and generic forms. DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. Doctors prescribe gabapentin to treat epilepsy, restless legs syndrome, and some types of nerve pain. Learn more the drug's uses, risks, and safety here. Other approved uses include fibromyalgia and restless legs syndrome. Gabapentin was first approved in 1993 and pregabalin was first approved in 2004. Gabapentin was first approved by the U.S. Food and Drug Administration (FDA) for the treatment of seizures in 1993 and was subsequently approved for one pain indication, postherpetic neuralgia. Gabapentin is a valuable medication for managing nerve pain and seizures, with a range of approved uses and benefits. While it is generally well-tolerated when used correctly, it is essential to be aware of its potential for dependence and significant side effects (like drowsiness and dizziness), as well as crucial drug interactions (especially There are rare postmarketing reports of individuals experiencing withdrawal symptoms shortly after discontinuing higher than recommended doses of gabapentin used to treat illnesses for which the drug is not approved. There are rare postmarketing reports of individuals experiencing withdrawal symptoms shortly after discontinuing higher than recommended doses of gabapentin used to treat illnesses for which the drug is not approved. In December 1993, the US Food and Drug Administration (FDA) granted approval for gabapentin, under the brand name Neurontin, for adjunctive therapy of partial seizures. Subsequently, the FDA approved gabapentin in 2000 for treatment of partial seizures in children aged 3 years or older and in 2002
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 |  |
 |  |
 |  |