Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
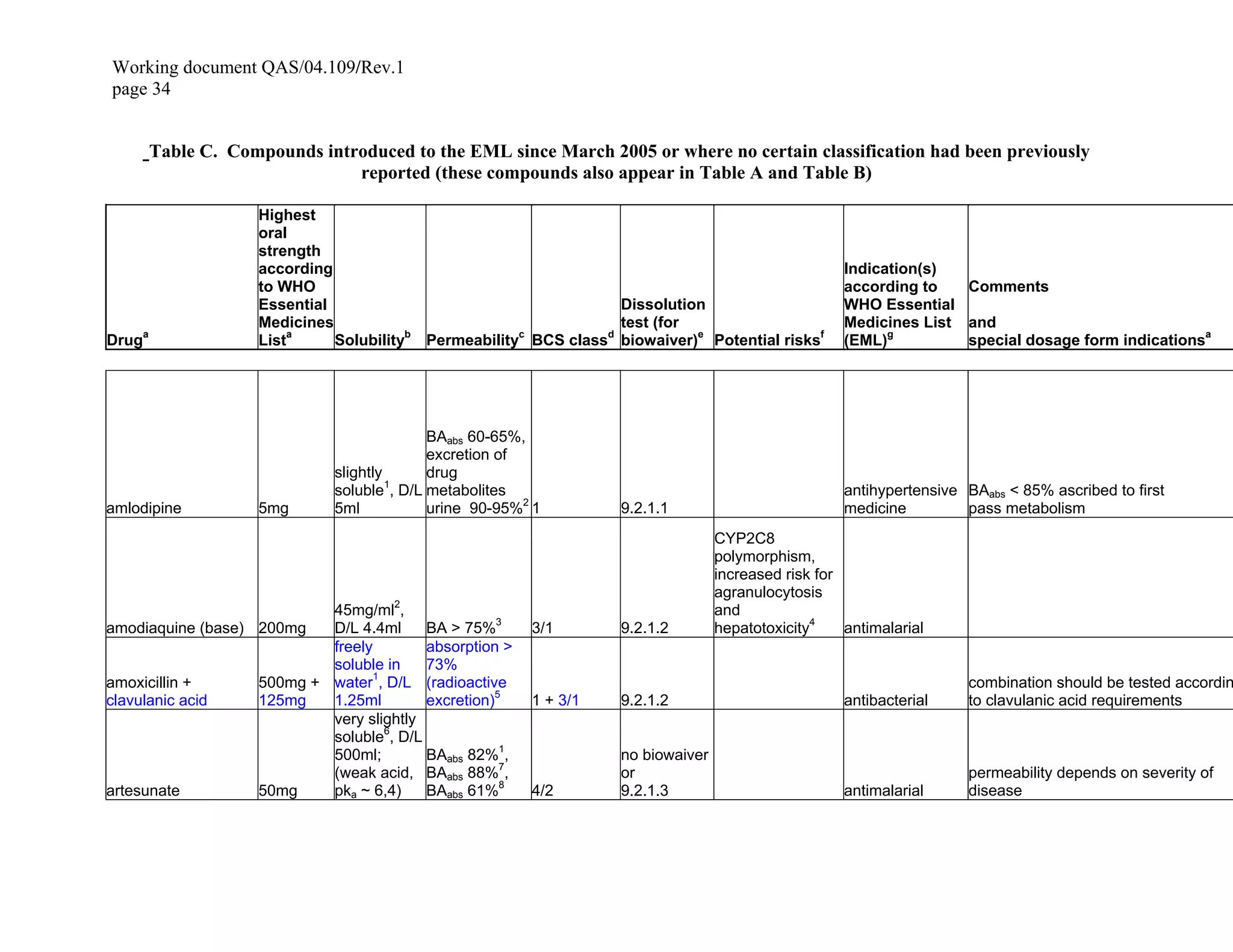
According to Biopharmaceutics Classification System (BCS), gabapentin enacarbil is classified as a BCS Class 2 compound which have a low solubility and a high permeability. In the BCS system, levetiracetam, gabapentin, and vigabatrin are classified as BCS class 1 drugs (39). These compounds are completely absorbed, with the exception of gabapentin that is about 70% absorbed in humans (40), although quite slowly. Case I: anti-epileptic drugs considers BCS classification that can have a significant effect on absorption. BCS class II (carbamazepine, lamotrigine and phenytoin) and BCS class III (gabapentin For example, Gabapentin, a structural analog of gamma-aminobutyric acid (GABA), a BCS class III drug, has permeability issues that lead to bioavailability problems. To overcome these issues, gabapentin is chemically conjugated with phosphatidylcholine and loaded as nanostructured lipid carriers (NLCs), which can be targeted to Phospholipase A2. The biopharmaceutics classification system (BCS)-based biowaiver approach is intended to reduce the need for in vivo bioequivalence studies, i.e., it can provide a surrogate for in vivo According to the biopharmaceutical classification system (BCS), Gabapentin is considered a class III drug. Its solubility is independent on the pH 21. The BCS (Biopharmaceutics Classification System)-based biowaiver approach is intended to reduce the need for in vivo bioequivalence studies i.e., it can provide a surrogate for in vivo bioequivalence. The Biopharmaceutical Classification System (BCS) has been a prognostic tool for assessing the potential effects of formulation on the human drug oral bioavailability. When used in conjunction with in vitro dissolution tests, the BCS can support the prediction of in vivo product performance and the development of mechanistic models that support formulation assessments through the generation of The effect of decreased renal function on gabapentin pharmacokinetics and dose recommendations was addressed by the sponsor using prior knowledge of effect of renal impairment on gabapentin clearance and a pharmacokinetic model for gabapentin concentrations after administration of G-ER formulation in healthy subjects. Pharmacologic Class: Gabapentin Enacarbil (XP13512) is a prodrug of gabapentin which belongs to an evolving class of compounds known as gabapentinoids or alpha2delta ligands. Gabapentin was encapsulated in PLGA nanoparticles as it belongs to BCS class III, with low solubility and low permeability thus make it a suitable choice for delivering via surface modified nanoparticles so as to increase bioavailability. Chitosan coated PLGA nanoparticle loaded with gabapentin were prepared by nanoprecipitation method. The biopharmaceutical classification system (BCS) classifies compounds based on their solubility and permeability. Regulatory agencies and health organizations have utilized this classification system to allow dissolution to be used to establish No new clinical pharmacology information was submitted in this NDA. Pregabalin has been determined to be a Biopharmaceutical Classification System (BCS) Class 1 compound with high solubility/high permeability characteristics (see Clinical Pharmacology review for NDA 21-446 by Dr. Sue Chih Lee dated 3/22/04 for additional details on this aspect and other relevant Clinical Pharmacology A platform lead by pharmaceutical specialists to grow-up pharmaceutical professionals with scientific and technical knowledge. Abstract The Biopharmaceutics Classification System (BCS) is an essential tool in pharmaceutical sciences, providing a scientific framework for classifying drugs based on their solubility and permeability characteristics. This system, developed in 1995, helps streamline drug development and regulatory approval processes by predicting in vivo drug performance from in vitro data. This paper Gabapentin enacarbil is classified as a BCS Class 2 compound (low solubility, high permeability). (b) (4) The applicant provided adequate information regarding structure elucidation and confirmation, method of manufacture, in-process controls, test methods, container closure system, and stability testing of gabapentin enacarbil drug substance. The major advances associated with the BCS concern the extensive work on dissolution of poorly absorbed BCS class II drugs in nutritional liquids (e.g. milk, peanut oil) and biorelevant media for the accurate prediction of the rate and the extent of oral absorption. The Biopharmaceutics Classification System (BCS) of drugs has been developed primarily to provide a scientific approach for clas- sifying oral immediate release drug formulations based on aque- The Biopharmaceutical Classification System (BCS) has been a prognostic tool for assessing the potential effects of formulation on the human drug oral bioavailability. When used in conjunction with in vitro dissolution tests, the BCS can support the What is BCS classification? BCS classification system is a scientific framework to differentiate the drug substances on the basis of solubility and permeability under prescribed condition. Where the solubility classification based on a United States Pharmacopoeia (USP) aperture and the intestinal permeability classification is based on a comparison to the intravenous injection. According to
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |