Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
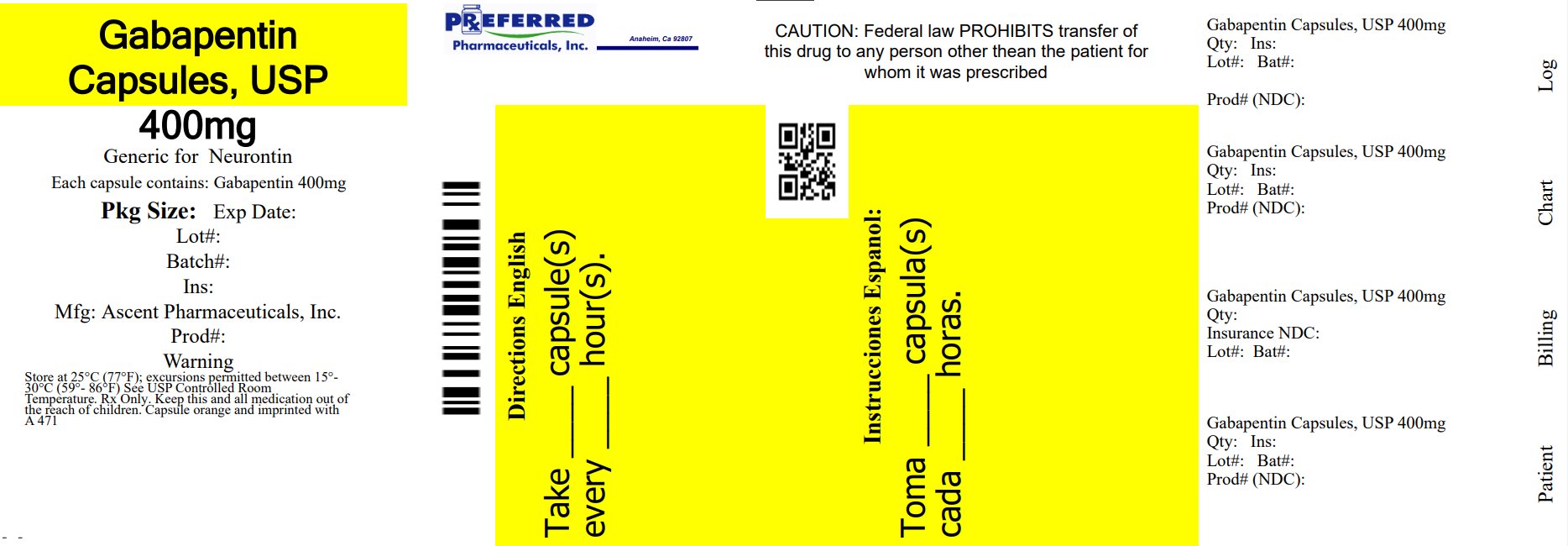
The present patent application discloses a method for the preparation of [ (1-aminomethyl)cyclohexyl]acetic acid (Gabapentin) by means of the Hofmann re-arrangement reaction and the extraction of the Gabapentin from the reaction mixture with a higher aliphatic alcohol, wherein the Hofmann reaction is conducted continuously, for the purpose of obtaining an active ingredient with a good yield The present invention is generally directed to methods for preparing stable gabapentin tablets by wet granulation. A wet granulation method for preparing gabapentin tablets includes forming a mixture by dry mixing of a first portion of a binder with the gabapentin, one or more excipients, or a combination of the gabapentin and the one or more excipients; and adding a second portion of the in which CS is the concentration, in mg per mL, of USP Gabapentin RS in the Standard preparation; C U is the concentration, in mg per mL, of gabapentin in the Assay preparation, based on the label claim; and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively. Gabapentin is available as oral capsules of 100 mg, 300 mg, and 400 mg; tablets of 100 mg, 300 mg, 400 mg, 600 mg, and 800 mg; and an oral solution of 50 mg/mL. However, the need often exists for a higher-concentration oral liquid, and if tablets or capsules are used as the drug source, a suspension will result. The present invention comprises an organic acid salt of gabapentin, wherein the organic acid is tartaric acid, ethanedisulfonic acid, or maleic acid. Methods for modulating the solubility and dose response of gabapentin are discussed. Methods of making organic acid salts of gabapentin are also discussed. Preparation method of gabapentin mouth dissolving Tablet [14] The direct compression method and a super dissolving agent, such as cross povidone, were used to create gabapentin tablets. After screening using a 40-mesh screen, the drug, super-disintegrant, diluents, and sweetening agent were appropriately mixed. The invention relates to a gabapentin capsule and a preparation method thereof. The gabapentin capsule comprises the following components: 150-300 g of gabapentin, 100-600 g of low substituted hydroxy propyl cellulose, 50-100 g of cross-linking polyvidone, 20-50 g of dodecyl sodium sulphate, 10-50 g of hydroxypropyl methylcellulose and 2-8 g of magnesium stearate. The gabapentin capsule The invention provides a gabapentin capsule preparation, which is a capsule directly filled with gabapentin bulk drug; the particle size distribution of the gabapentin raw material medicine is 50-70 meshes. The invention provides a simpler gabapentin capsule preparation technology, and toxic and side effects brought by auxiliary materials or degradation products are greatly reduced. Standard preparation—Dissolve an accurately weighed quan-tity of USP Gabapentin RS in Diluent, and dilute quantitatively, and stepwise if necessary, with Diluent to obtain a solution hav- ing a known concentration of about 14.0 mg per mL. Assay preparation—Transfer about 350 mg of Gabapentin, accurately weighed, to a 25-mL volumetric flask, dissolve in and dilute with Diluent to volume Preparation of standard calibration curve of Gabapentin entin (10 mg) was dissolved in 10 ml of in pH 1.2 0.1 N HCl and volume was made up to 100 ml in 100 ml volumetric flask. This solution (100 mcg / ml) was further dilute Gabapentin package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. For example, Gabapentin, a structural analog of gamma-aminobutyric acid (GABA), a BCS class III drug, has permeability issues that lead to bioavailability problems. To overcome these issues, gabapentin is chemically conjugated with phosphatidylcholine and loaded as nanostructured lipid carriers (NLCs), which can be targeted to Phospholipase A2. Field of Invention [0001] The present invention relates to an improved process for the preparation of Gabapentin. The process also relates to a new process for the preparation of 1,1-cyclohexane diacetic acid monoamide (CDMA), which is a key intermediate for the preparation of Gabapentin. US-A-6054482 discloses that the preparation and long-term storage of gabapentin and its pharmaceutically acceptable salts present problems since (i) during the preparation the compounds show considerable variations without apparent reason; (ii) very pure gabapentin, when stored long term, shows differing stabilities; and (iii) a toxic lactam Because GABAPENTIN CAPSULES is eliminated solely by renal excretion, dosage adjustments are recommended for patients with renal impairment (including elderly patients with declining renal function) and patients undergoing hemodialysis (See DOSAGE AND ADMINISTRATION, Special Patient Populations, Table 2 and WARNINGS AND PRECAUTIONS, Neurologic). The objective of the present study was to develop a pharmaceutically equivalent, stable, robust, cost effective and quality improved formulation of Gabapentin controlled release tablets by using Formulation Reviewed: February, 2021 Disclaimer: The information in this compounding and preparation worksheet was developed for in-house use only by The Hospital for Sick Children (“SickKids”) and its staff. A process for preparation of gabapentin comprising a step of obtaining 1,1-cyclohexane diacetic acid monoamide from 1,1-cyclohexane diacetic acid anhydride, wherein said reaction is characterized by the use of ammonia precursor or pre-generated ammonia-isopropanol solution. The invention further discloses preparation of gabapentin and isolation of gabapentin in polymorphic Form II with high Procedure: Empty capsule contents into a mortar. Using a pestle, grind to a very FINE powder for a minimum of 10 minutes. NEW** Add a small amount of vehicle to powder and let soak for 60 minutes. NEW** Levigate into a uniform paste using pestle. in which CS is the concentration, in mg per mL, of USP Gabapentin RS in the Standard preparation; C U is the concentration, in mg per mL, of gabapentin in the Assay preparation, based on the label claim; and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |