Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
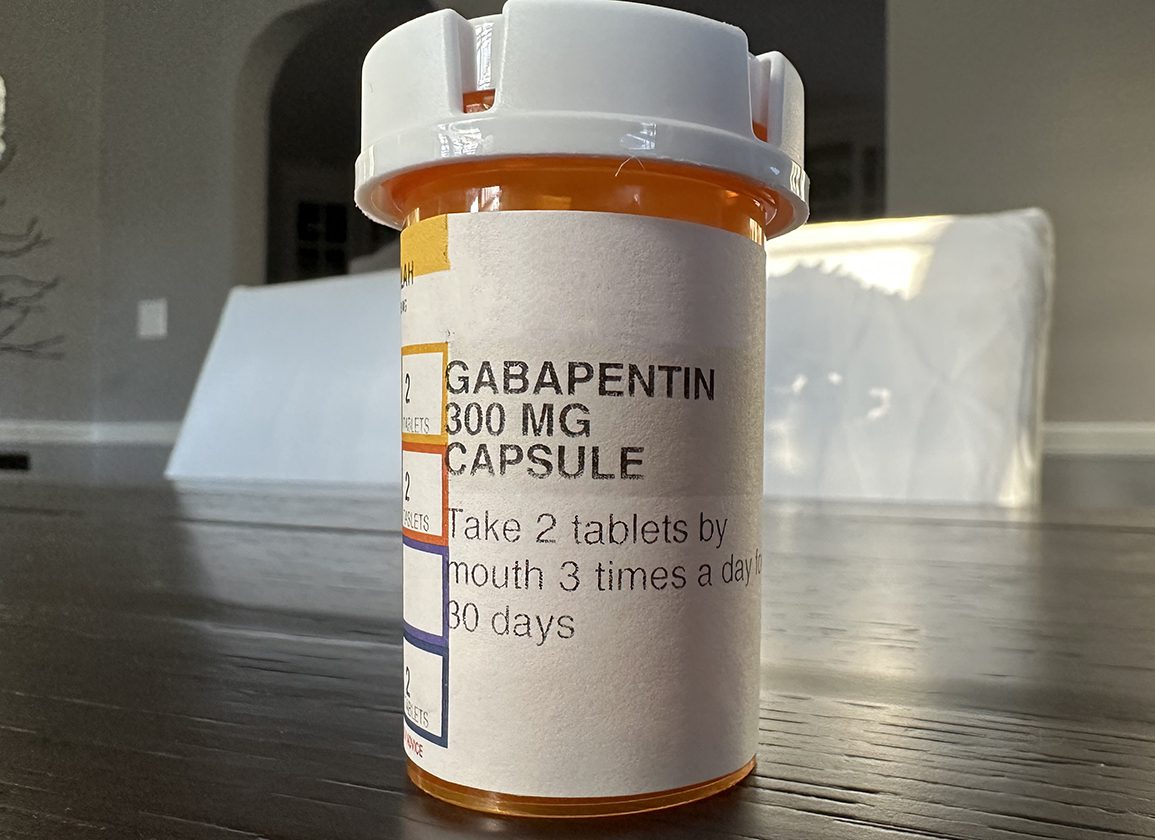
Gabapentin — an anti-seizure drug also approved for nerve pain related to shingles — is now among the most widely prescribed drugs in the country. It's used off-label to treat menopausal hot In December 1993, the US Food and Drug Administration (FDA) granted approval for gabapentin, under the brand name Neurontin, for adjunctive therapy of partial seizures. Subsequently, the FDA approved gabapentin in 2000 for treatment of partial seizures in children aged 3 years or older and in 2002 FDA is warning that serious breathing difficulties may occur in patients using gabapentin or pregabalin who have respiratory risk factors. Gabapentin gained FDA approval in 1993 under the brand name Neurontin as an adjunctive therapy in the treatment of partial onset seizures, and subsequently for the treatment of postherpetic neuralgia in adults in 2002. It became available as a generic in 2004. Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. However, it was later discovered that gabapentin has the potential of an anticonvulsive medication and can be used as an adjunct to more Gabapentin and pregabalin are FDA-approved for a variety of uses include fibromyalgia and restless legs syndrome. Gabapentin was first approved in 1993 and pregabalin was Gralise (gabapentin) Tablets 300 mg and 600 mg Company: Abbott Products, Inc. Application No.: 022544 Approval Date: 01/28/2011 Persons with disabilities having problems accessing the PDF files below may call (301) 796-3634 for assistance. Approval Letter (s) (PDF) Summary Review (PDF) Officer/Employee List (PDF) Cross Discipline Team Leader Gabapentin, sold under the brand name Neurontin among others, is an anticonvulsant medication primarily used to treat neuropathic pain and also for partial seizures [10][7] of epilepsy. It is a commonly used medication for the treatment of neuropathic pain caused by diabetic neuropathy, postherpetic neuralgia, and central pain. [11] It is moderately effective: about 30–40% of those given DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. Gabapentin has been approved by the United States (US) Food and Drug Administration (FDA) for postherpetic neuralgia and as adjunctive therapy for focal seizures. 1 However, a recent analysis of US physician office-based prescription practices between 2011 and 2016 found that less than one percent of gabapentin prescriptions are for such indications. 2 In 2020, gabapentin was reported to be Gabapentin is an anticonvulsant (antiseizure) medication approved by the FDA to treat several conditions. Doctors sometimes prescribe gabapentin "off-label" to treat other conditions as well. A 2022 report stated that gabapentin was among the 10 most commonly prescribed medications in the U.S. What is gabapentin and what is it used for? Gabapentin is used to control seizures, to treat nerve DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. Gabapentin is from a group of medicines called anticonvulsants. Different brands of gabapentin are not interchangeable and they are FDA approved for different conditions. Use only the brand and form of gabapentin your doctor has prescribed. Check your medicine each time you get a refill to make sure you receive the correct form. NEURONTIN® (gabapentin) capsules, for oral use NEURONTIN® (gabapentin) tablets, for oral use NEURONTIN® (gabapentin) oral solution Initial U.S. Approval: 1993 NEURONTIN® (gabapentin) capsules, for oral use NEURONTIN® (gabapentin) tablets, for oral use NEURONTIN® (gabapentin) oral solution Initial U.S. Approval: 1993 NEURONTIN® (gabapentin) capsules, for oral use NEURONTIN® (gabapentin) tablets, for oral use NEURONTIN® (gabapentin) oral solution Initial U.S. Approval: 1993 Approval Date: 10/12/2000 Approval Letter (s) (PDF) Printed Labeling (PDF) Medical Review (s) Part 1 (PDF) Part 2 (PDF) Pharmacology Review (s) (PDF) Statistical Review (s) (PDF) Clinical Pharmacology Biopharmaceutics Review (s) (PDF) Administrative Document (s) & Correspondence (PDF) Date created: May 062004 Back to Top Drugs@FDA GABAPENTIN (Trade Name: Neurontin®) Introduction: Gabapentin is a prescription medication approved by the United States Food and Drug Administration (FDA) for the treatment of neuropathic pain and epileptic disorders. This drug is currently marketed in capsule, tablet, and oral solution formulations. FDA approval history for Gralise (gabapentin) used to treat Postherpetic Neuralgia. Supplied by Depomed, Inc. Gabapentin was first approved by the U.S. Food and Drug Administration (FDA) for the treatment of seizures in 1993 and was subsequently approved for one pain indication, postherpetic neuralgia.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |