Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 |  |
 |  |
 |  |
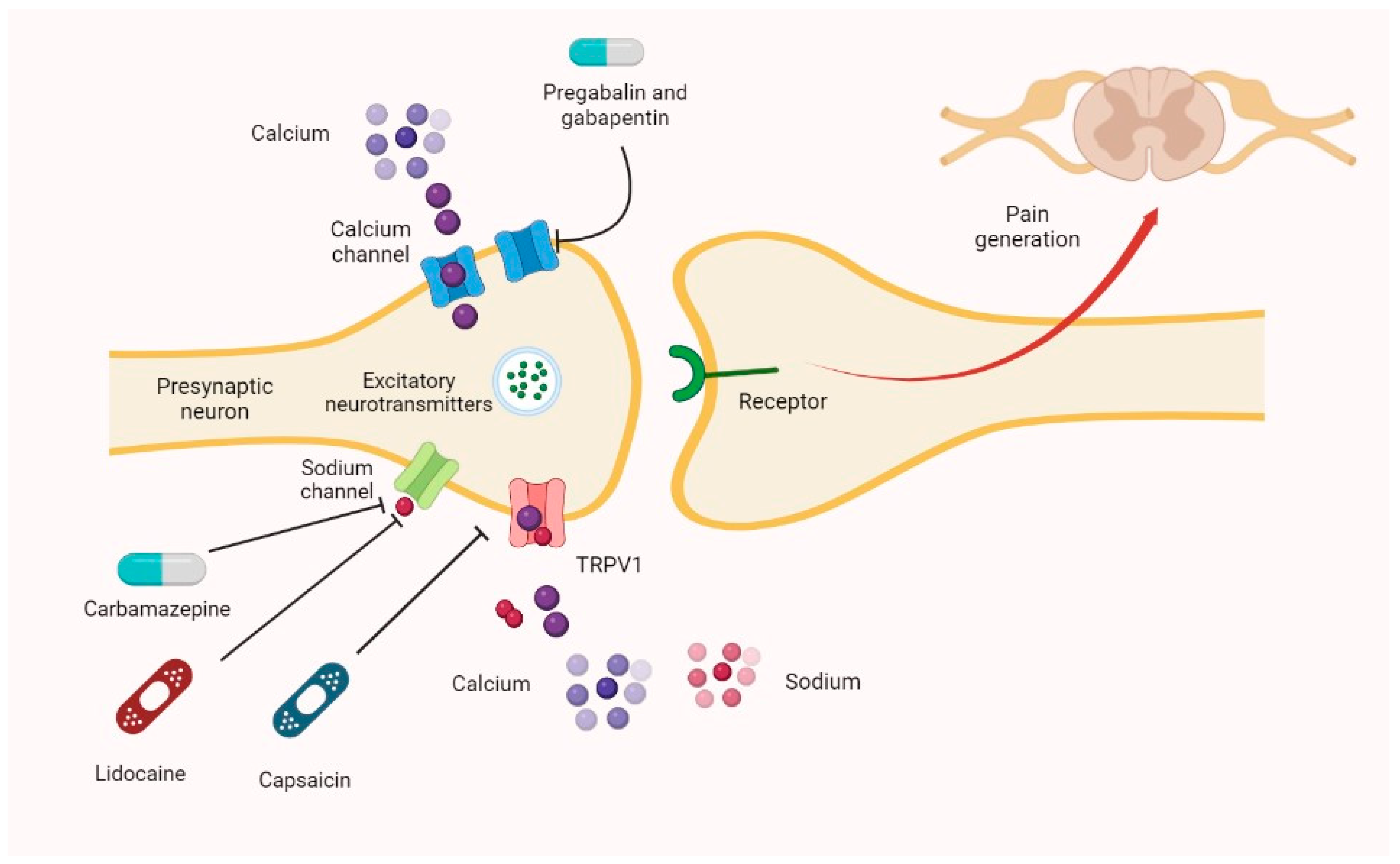
Gabapentin's elimination involves renal clearance, with minimal metabolism by the liver. Its half-life is typically 5-7 hours, but can vary based on renal function. Clinical factors like dosage, individual metabolism, and renal impairment can influence its clearance. Summary: This paper describes the pharmacokinetic studies of 1-(aminomethyl)-cyclohexane acetic acid (gabapentin, Go 3450, CI-945) conducted with the '4C-labelled substance fal-lowing intravenous and intragastric administration to rats and dogs and oral administration to humans. Gabapentin is conspicuous among anticonvulsant drugs for its lack of clinically relevant drug interactions, because of the lack of hepatic metabolism and ability to induce or␣inhibit hepatic microsomal enzymes, and low protein binding. Pharmacodynamics Mechanisms of action Gabapentin and pregabalin do not bind to GABA receptors despite their structural similarity but have a high affinity for the α2δ-1 subunit of voltage-gated Gabapentin | Deranged PhysiologyGabapentin Gabapentin is not protein-bound. A high volume of distribution indicates greater concentration in tissue than in plasma. It is not metabolized and does not induce hepatic enzymes or inhibit metabolism of other antiepileptic drugs. Absorption of gabapentin is solely dependent on LAT that are easily saturable, resulting in dose-dependent pharmacokinetics. As the dose of gabapentin increases, the area under the plasma concentration–time curve (AUC) does not increase proportionally. Metabolism: In humans, gabapentin undergoes minimal metabolic alteration, largely retaining the original structure. Gabapentin does not induce or inhibit CYP enzymes. Gabapentin (Trade name: Neurontin) is an anticonvulsant. It is commonly also used off-label for anxiety disorders, restless leg syndrome, and in alcohol use disorder. In vitro studies were conducted to investigate the potential of gabapentin to inhibit the major cytochrome P450 enzymes (CYP1A2, CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4) that mediate drug and xenobiotic metabolism using isoform selective marker substrates and human liver microsomal preparations. Although both gabapentinoids are absorbed in the small intestine, pregabalin is also absorbed in the proximal colon. Absorption of gabap-entin is solely dependent on LAT that are easily satura-ble, resulting in dose-dependent pharmacokinetics. Gabapentin is approved for the treatment of postherpetic neuralgia (PHN) and epilepsy. The pharmacokinetic (PK) properties of gabapentin, including absorption, distribution, metabolism, and excretion (ADME), were investigated during the development of Neurontin®, an immediate-release (IR) formulatio T 4 is slowly eliminated through its major metabolic pathway to T 3 via sequential deiodination, where approximately 80% of circulating T 3 is derived from peripheral T 4. Gabapentin binds to the α2δ-1 subunit of voltage-gated calcium channels in the CNS, particularly in presynaptic neurons. The inhibition of neurotransmitter release leads to dampened neuronal hyperexcitability, especially in epileptic foci and pain pathways. In addition, gabapentin has a relative lack of drug–drug interactions, which makes it a desirable adjunctive agent in the management of epilepsy in patients taking multiple medications. A low degree of protein binding and freedom from hepatic metabolism also make it an attractive agent in patients with hepatic impairment. Gabapentin is a new antiepileptic drug (AED) with an attractive pharmacokinetic profile. It is absorbed by an active and saturable transport system, and has a high volume of distribution. Gabapentin is not bound to plasma proteins, does not induce hepatic enzymes and is not metabolized. At steady st The new antiepileptic medications are prescribed for the treatment of patients with seizure disorders since 17 years ago. Gabapentin (GBP) was approved on January 1994 as adjunctive treatment in patients 12 years or older with partial seizures, with Orally administered gabapentin exhibits saturable absorption--a nonlinear (zero-order) process--making its pharmacokinetics less predictable. Plasma concentrations of gabapentin do not increase proportionally with increasing dose. Gabapentin was tested for its effects on seven enzymes in the metabolic pathways of these two neurotransmitters: alanine aminotransferase (AL-T), aspartate aminotransferase (AS-T), GABA aminotransferase (GABA-T), branched-chain amino acid aminotransferase (BCAA-T), glutamine synthetase (Gln-S), glutaminase (GLNase), and glutamate dehydrogenase (
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 |  |
 |  |
 |  |