Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
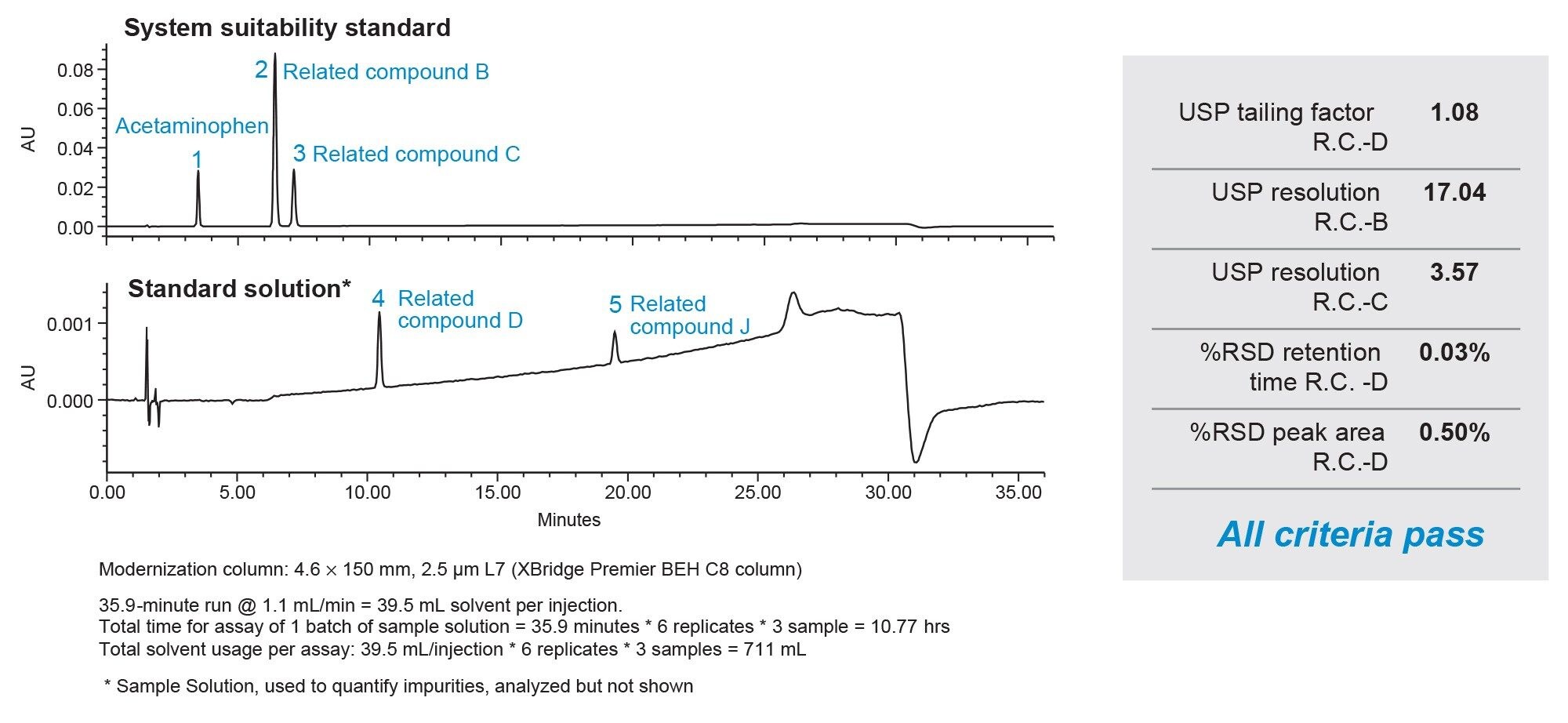
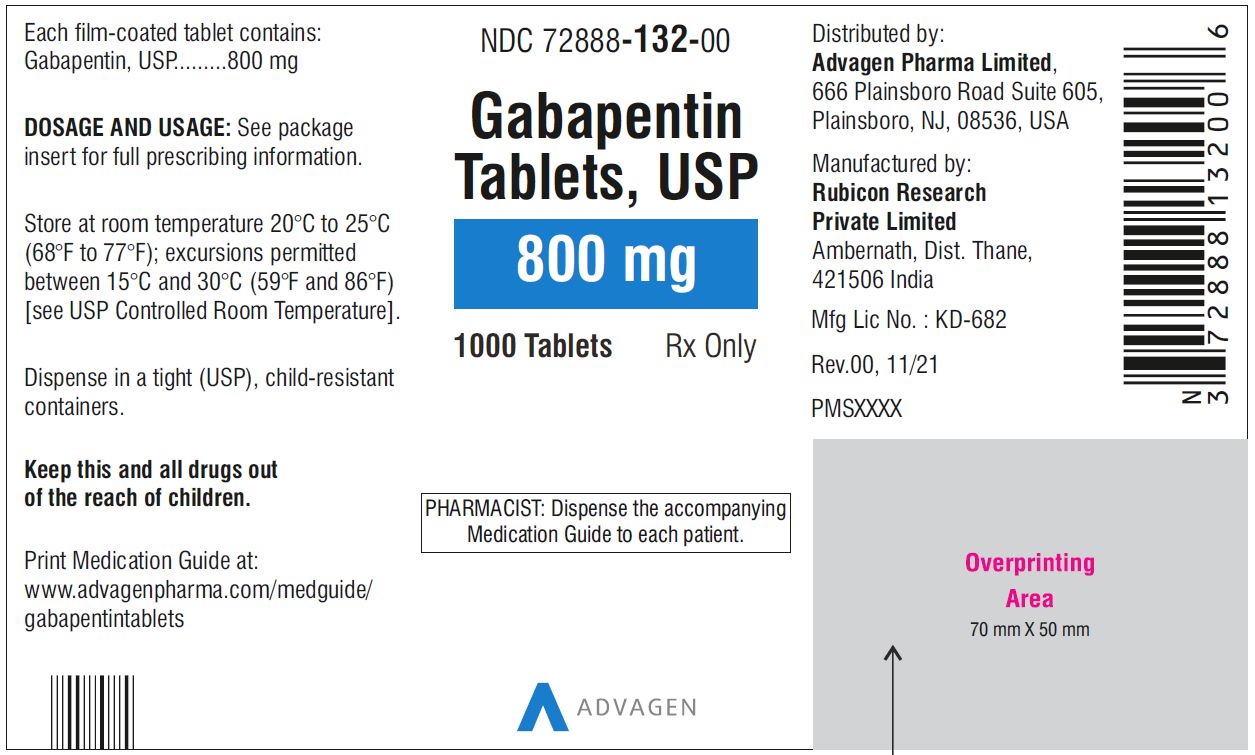
PRODUCT MONOGRAPH PrTEVA-GABAPENTIN Gabapentin Capsules 100 mg, 300 mg, and 400 mg Tablets 600 mg and 800 mg Antiepileptic Agent NEURONTIN (gabapentin capsules 100 mg, 300 mg and 400 mg; gabapentin tablets 600 mg and 800 mg), submission control 274395, Product Monograph, BGP Pharma ULC (MAY 05, 2023) USP 35 Gabapentin RS, USP Gabapentin Related Compound A RS, and USP Gabapentin Related Compound B RS, respectively. Test solution—Use the Assay preparation. Standard solution—Dissolve a suitable quantity of USP Gabapentin Related Compound E RS in Diluent to obtain a solu-tion having a known concentration of 8.4 mg per mL. Chromatographic system (see Chromatography á621ñ)—Pre-pare as Information on drug and health products authorized by Health Canada. Gabapentin 1 in which rU and rS are the peak responses for the Test solution and the Working standard solution, respectively; CS is the concentra-tion, in mg per mL, of the Working standard solution; 900 is the volume, in mL, of Medium; 100 is the conversion factor to percent-age; and L is the Tablet label claim, in mg. Tolerances—Not less than 80% (Q) of the labeled amount of gabapentin Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. However, it was later discovered that gabapentin has the potential of an anticonvulsive medication and can be used as an adjunct to more PRODUCT MONOGRAPH Pr NEURONTIN® Gabapentin Capsules 100 mg, 300 mg, and 400 mg Tablets 600 mg and 800 mg Antiepileptic Agent Pfizer Canada ULC Date of Revision: 17,300 Trans-Canada Highway January 27, 2020 Kirkland, Quebec H9J 2M5 www.pfizer.ca Submission Control No: 232770 ®TM Warner-Lambert Company LLC Pfizer Canada ULC, Licensee ©Pfizer Canada ULC 2019 NEURONTIN® (gabapentin) Product Home - British Pharmacopoeia This leaflet is part III of a three-part "Product Monograph" published when GABAPENTIN was approved for sale in Canada and is designed specifically for Consumers. The active ingredient in gabapentin capsules, USP is gabapentin, which has the chemical name 1- (aminomethyl)cyclohexaneacetic acid. The molecular formula of gabapentin is C9H17NO2 and the 1 NEURONTIN® (Gabapentin Capsules, 100 mg, 300 mg and 400 mg; Gabapentin Tablets, 600 mg and 800 mg), submission control 275525, Product Monograph, BGP Pharma ULC. DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. GABAPENTIN (gabapentin) is indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. GabapentinGabapentin ₹5,900 Ex Tax: ₹5,000Qty Add to Cart United States Pharmacopeia (). USP Monographs, Gabapentin. USP-NF. Rockville, MD: United States Pharmacopeia. RIVA-GABAPENTIN étant principalement éliminé par voie urinaire, un réglage posologique peut s’avérer nécessaire chez les patients âgés, en raison de la diminution de leur fonction rénale (voir 4.2 Posologie recommandée et ajustement posologique; 10.3 Pharmacocinétique, Populations particulières et états pathologiques). A comprehensive guide to gabapentin, an anticonvulsant and analgesic drug. Includes indications, dosage, warnings, interactions, stability, and more. This web page provides the label of Neurontin (gabapentin), a drug used for epilepsy and neuropathic pain. It includes information on gabapentin's mechanism of action, pharmacokinetics, dosage, and adverse reactions. NEURONTIN (gabapentin) is indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. Gabapentin is not metabolized to a significant extent in humans. Gabapentin does not induce or inhibit hepatic mixed function oxidase enzymes responsible for drug metabolism and does not interfere with the metabolism of commonly co-administered antiepileptic drugs.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |