Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
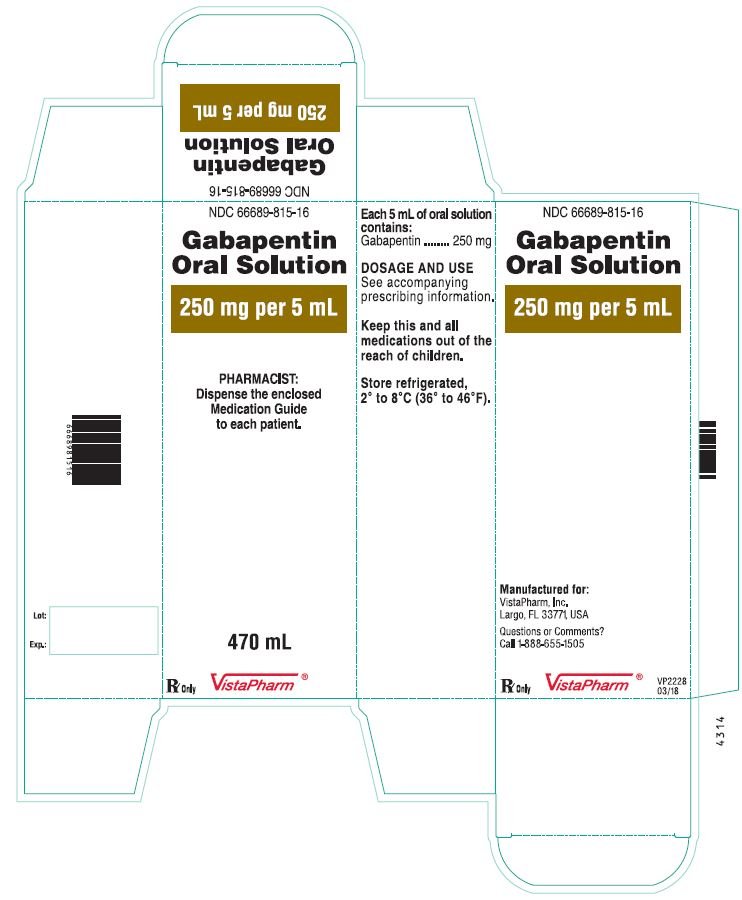 |  |
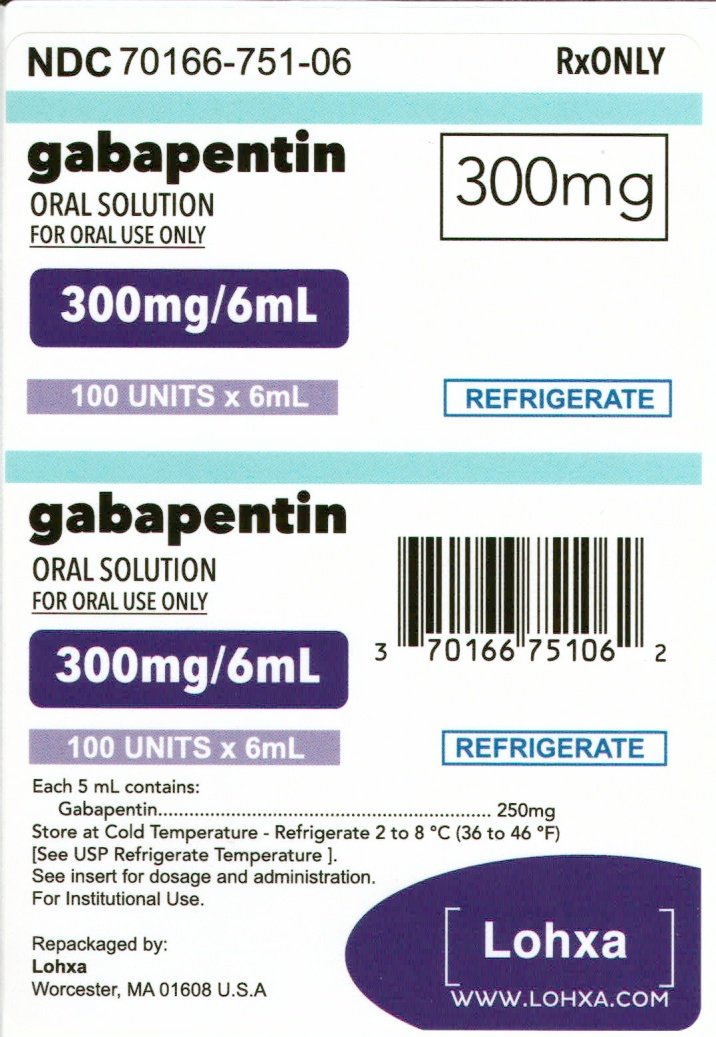
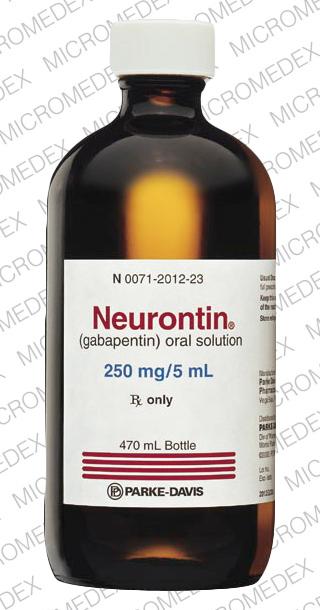
NEURONTIN oral solution contains 250 mg of gabapentin per 5 mL (50 mg per mL) and the following inactive ingredients: glycerin, xylitol, purified water, and artificial cool strawberry anise flavor. Gabapentin Oral Solution package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. Gabapentin Oral Solution Stability at Room Temperature Gabapentin oral solution is stable at room temperature for up to 24 hours, according to the American Society of Health-System Pharmacists. After this time frame, it begins to degrade and should be discarded and replaced with a fresh dose. It is important that Gabapentin oral solution not be stored above 25°C (77°F). Liquid Gabapentin Not DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. Who should not take Gabapentin Oral Solution? n Oral Solution. See the end of this Medication Guide for a complete list of ingredients in Gabapent What should I tell my healthcare provider before taking gabapentin? Before taking gabapentin, tell your healthcare provider if you: gabapentin 100 mg/mL Oral Suspension Batch No: Additional Information: If suspension settles on bottom of bottle and can’t be shaken back into suspension then remove cap, stir with stirring rod, replace cap, shake bottle well, measure dose. Typically, liquid gabapentin should be refrigerated and can be stable at room temperature for a limited period. Inspect the Solution: Look for any changes in color, clarity, or consistency. If you notice any unusual signs such as cloudiness or sediment, it’s best to err on the side of caution. In adults with postherpetic neuralgia, gabapentin oral solution may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). The dose can subsequently be titrated up as needed for pain relief to a dose of 1800 mg/day (600 mg three times a day). When it comes to medication, proper storage is critical for maintaining efficacy and safety. One question that often comes up for patients and caregivers dealing with gabapentin liquid is: Does it need to be refrigerated? Understanding the storage requirements of this medication can save you from potential complications. In this comprehensive article, we will explore gabapentin liquid, its The active ingredient in Gabapentin Oral solution is gabapentin, which has the chemical name 1-(aminomethyl)cyclohexaneacetic acid. The molecular formula of gabapentin is C9H17NO2 and the molecular weight is 171.24. Stopping Gabapentin Oral Solution suddenly can cause serious problems. Gabapentin Oral Solution can cause serious side effects including: 1. Like other antiepileptic drugs, Gabapentin Oral Solution may cause suicidal thoughts or actions in a very small number of people, about 1 in 500. clarithromycin [Biaxin]). For more information on storage and stability of reconstituted medications see our chart, Pediatric Oral Antibiotic and Antifungal Suspensions and Liquids (Detail-Documents #231107 [U.S. subscribers] and #231122 [Canadian subscribers]) and our Technician Training Tutorial, Dispensing Pediatric Antibiotic Suspensions. Gabapentin is used with other medications to prevent and control seizures. It is also used to relieve nerve pain following shingles (a painful rash due to herpes zoster infection) in adults. Gabapentin is known as an anticonvulsant or antiepileptic drug. This study reports the stability of extemporaneously prepared gabapentin oral suspensions prepared at 100 mg/mL from bulk drug and capsules in either Oral Mix or Oral Mix SF suspending vehicles. Suspensions were packaged in amber plastic bottles and Gabapentin may be administered as the oral solution, capsule, or tablet, or using combinations of these formulations. Dosages up to 50 mg/kg/day have been administered in a long-term clinical study. The maximum time interval between doses should not exceed 12 hours. Page 7: Quality Care Products, LLC: Gabapentin is indicated for: Management of postherpetic neuralgia in adults. Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in Proper medication storage is important to ensure medication shelf life until the manufacturer expiration date and to reduce waste. Many meds are recommended to be stored at controlled-room temperature. However, several meds require storage in the refrigerator or freezer to ensure stability. See our toolbox, Medication Storage: Maintaining the Cold Chain, for helpful storage tips and other When the decision is made to co-prescribe Gabapentin Oral Solution with another CNS depressant, particularly an opioid, or to prescribe Gabapentin Oral Solution to patients with underlying respiratory impairment, monitor patients for symptoms of respiratory depression and sedation, and consider initiating Gabapentin Oral Solution at a low dose. Neurontin® (gabapentin) Capsules, Neurontin® (gabapentin) Tablets, and Neurontin® (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. Gabapentin oral solution is a commonly prescribed medication used to treat various conditions such as epilepsy, neuropathic pain, and restless leg syndrome.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |