Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
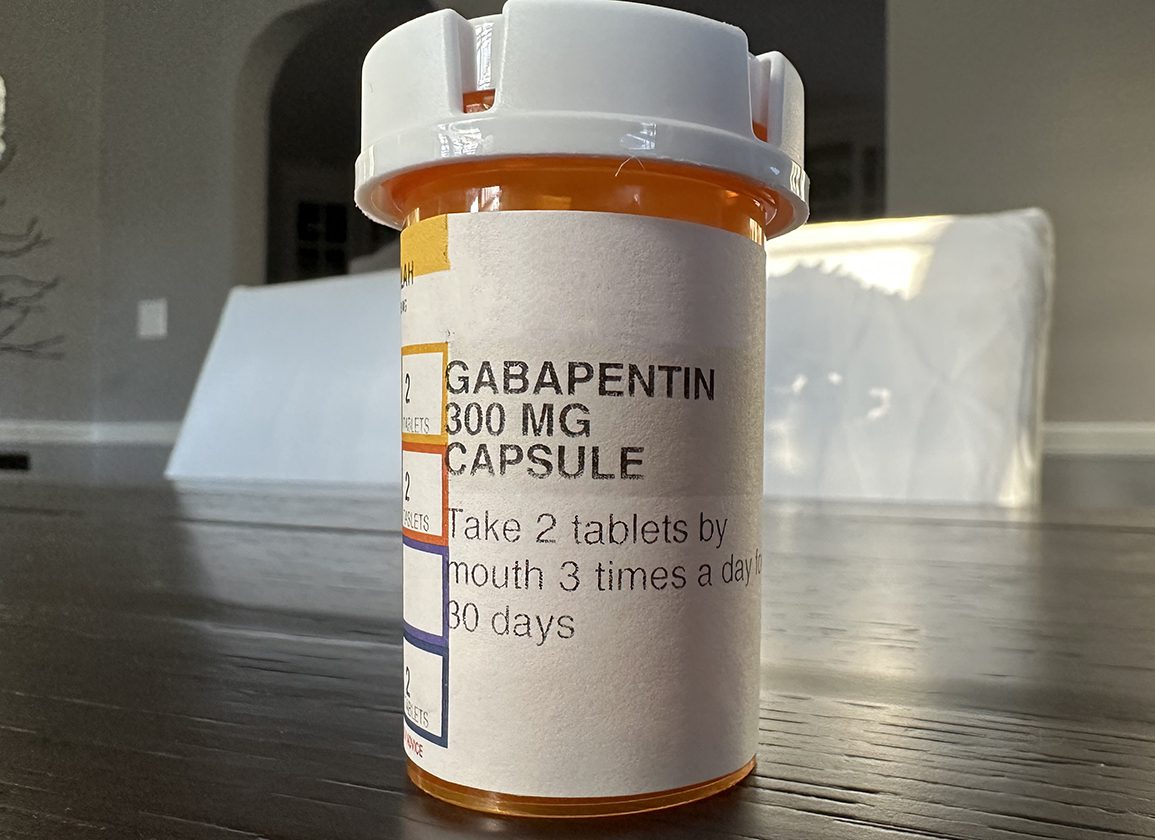
This bill amends Montana's drug scheduling law to add gabapentin to Schedule V controlled substances, which is a category of drugs with lower potential for abuse. ver view ective May 1, 2024, all Gabapentin products will be Schedule V controlled substances in Utah. For schedules, the rule changes adopt the federal schedule subject to drugs scheduled by the state after January 6, 2022, and the rules promulgated by the Michigan Board of Pharmacy; remove Brorphine, Gabapentin, and Pentazocine as exceptions to the federal schedule; provide an exception to the federal scheduling for isomers, Salvia Divorum As part of new changes to the medical fee schedule, this rule will take effect January 1, 2025. Gabapentin isn’t classified as a controlled substance by the federal government. However, some states recognize it as a Schedule V controlled substance and regulate its use. This is because of the growing concerns about misuse and abuse of the drug. In seven states, gabapentin is classified as a schedule V controlled substance (including AL, KY, MI, ND, TN, VA, and WV). Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). By placing gabapentin under stricter control, the bill seeks to mitigate risks associated with its non-medical use and enhance public safety. Debate surrounding the bill has highlighted the balance between ensuring access to necessary medications for patients and preventing potential abuse. Generally revise dangerous drug schedule. Register now for our free OneVote public service or GAITS Pro trial account and you can begin tracking this and other legislation, all driven by the real-time data of the LegiScan API. Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules depending upon the drug’s acceptable medical use and the drug’s abuse or dependency potential. Gabapentin isn’t considered a controlled substance by the federal government as of July 2022. But several states consider gabapentin a schedule V (schedule 5) controlled substance. Gabapentin is a Schedule V drug in states where it’s classified as a controlled substance. Despite its increasing use, especially for off-label purposes, gabapentin typically does not have the same potential for misuse or dependence as some other drugs, such as opioids or benzodiazepines. Will Gabapentin prescription histories be available in the Controlled Substance Reporting System? • Yes, Gabapentin prescription histories will be available to practitioners with a Controlled Substances Reporting System (CSRS) registration. In accordance with a new state law, the anticonvulsant and nerve pain medication gabapentin will soon be added to the list of drugs tracked through the state’s prescription drug management program (PDMP), the NC Controlled Substances Reporting System (NC CSRS). While gabapentin remains a non-controlled substance, Session Law 2023-65 Part XI Section 11.1 G.S. 90-113.73 (b) adds it to the Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of gamma-aminobutyric acid (GABA). Will Gabapentin prescription histories be available in the Controlled Substance Reporting System? • Yes, Gabapentin prescription histories will be available to practitioners with a Controlled Substances Reporting System (CSRS) registration. Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Controlled substances: schedules; classification of gabapentin as a schedule 5 controlled substance; modify. Amends sec. 7201 of 1978 PA 368 (MCL 333.7201). Adds Gabapentin to the list of substances to be reported into the CSRS, by dispensers, effective March 1, 2024; this law requires veterinarians to report prescriptions of Gabapentin effective March 1, 2025. We would like to show you a description here but the site won’t allow us. Gabapentin’s regulatory status varies by state. Some states classify it as a Schedule V controlled substance due to concerns about misuse and its involvement in the opioid crisis. Others do not schedule it but require mandatory reporting to state prescription drug monitoring programs (PDMPs) to track prescribing and dispensing.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |