Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 |  |
 |  |
 |  |
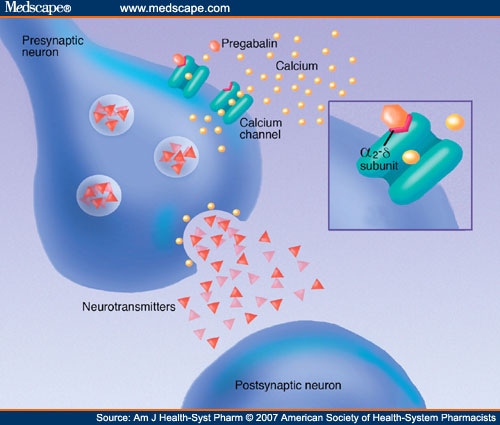
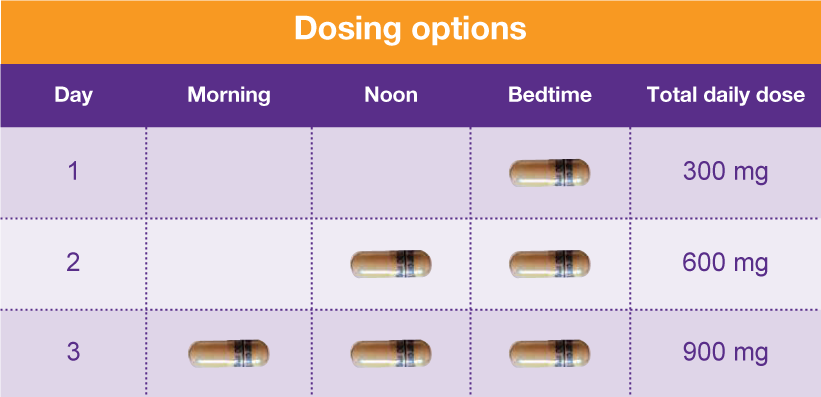
In accordance with the provisions of the Administrative Procedure Act (R.S. 49:950 et seq.) and the Pharmacy Practice Act (R.S. 37:1161 et seq.), the Louisiana Board of Pharmacy hereby gives notice of its intent to amend several sections of its chapter of rules for the state prescription monitoring program (PMP). The proposed changes for §2901 remove several terms and their definitions which 2011 Louisiana Laws Revised Statutes TITLE 40 — Public health and safety RS 40:964 — Composition of schedules Abstract Background: Between August 2016 and July 2018, three states classified gabapentin as a Schedule V drug and nine states implemented prescription drug monitoring program (PDMP) regulation for gabapentin. It is highly unusual for states to take drug regulation into their own hands. The impact of these changes on gabapentin prescribing is unclear. Gabapentin’s regulatory status varies by state. Some states classify it as a Schedule V controlled substance due to concerns about misuse and its involvement in the opioid crisis. Others do not schedule it but require mandatory reporting to state prescription drug monitoring programs (PDMPs) to track prescribing and dispensing. 20 Scholar using the search terms “gabapentin OR neurontin”, “gabapentin AND off-label”, 21 “gabapentin AND controlled substance”, “gabapentin AND substance use disorder” and 22 “gabapentin AND opioids”. Additional articles were identified by manual review of the reference 23 lists of pertinent publications. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation which contains any quantity of the following substances having a depressant effect on the central nervous system, including its salts, isomers, or salts of isomers, whenever the existence of such salts, isomers, and salts of There are established five schedules of controlled substances, to be known as Schedules I, II, III, IV, and V. Such schedules shall initially consist of the substances listed in R.S. 40:964. In determining that a substance is to be added to these schedules, the secretary shall find the following: A. As to Schedule I: This means gabapentin has a lower risk of abuse compared to Oxycontin (oxycodone), which is a schedule II opioid medication. At this time, seven states label gabapentin as a controlled substance. Gabapentin isn’t considered a controlled substance by the federal government as of July 2022. But several states consider gabapentin a schedule V (schedule 5) controlled substance. Between August 2016 and July 2018, three states classified gabapentin as a Schedule V drug and nine states implemented prescription drug monitoring program (PDMP) regulation for gabapentin. It is highly unusual for states to take drug regulation Individuals at the highest risk for abusing gabapentin include those with opioid abuse, mental illness, or previous history of prescription drug abuse. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. Gabapentin enacarbil ER (Horizant®) is a prodrug of gabapentin. Gralise and Horizant are indicated for the management of postherpetic neuralgia (PHN). Horizant is also indicated for the treatment of moderate-to-severe primary restless legs syndrome (RLS) in adults. Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. RS 40:978 - Prescriptions A. Except when dispensed or administered directly by a medical practitioner or administered by a person authorized to administer by such practitioner, other than a pharmacist, to an ultimate user, no controlled dangerous substance included in Schedule II, which is a prescription drug as determined under the Louisiana Revised Statutes of 1950, may be dispensed or Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of gamma-aminobutyric acid (GABA). RS 40:964 - Composition of schedules. Schedules I, II, III, IV, and V shall, unless and until added pursuant to R.S. 40:962, consist of the following drugs or other substances, by whatever official name, common or usual name, chemical name, or brand name designated: SCHEDULE I. A. Opiates. The increase is a direct result of the additional reporting requirement of two drugs of concern (promethazine in oral liquid formulations and gabapentin containing products) as of January 20, 2021. There is a continued downward trend in the number of opioid and controlled dangerous substance prescription dispensations to Louisiana patients. Gabapentin is classified as a controlled substance in several states, including Alabama, Georgia, Kentucky, Tennessee, and Texas. These states have placed it under Schedule V, indicating a lower potential for abuse compared to higher schedules. In addition, a federal rule became effective August 18, 2014 which places tramadol (Ultram) products into Schedule IV of the federal list of controlled substances. This product was previously a non-controlled substance and can no longer be prescribed by APRNs in Louisiana for the management of chronic, non-cancer related pain. D. As to Schedule IV: (1) The drug or other substance has a low potential for abuse relative to the drugs or other substances listed in Schedule III. (2) The drug or other substance has a currently accepted medical use in treatment in the United States, and
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 |  |
 |  |
 |  |